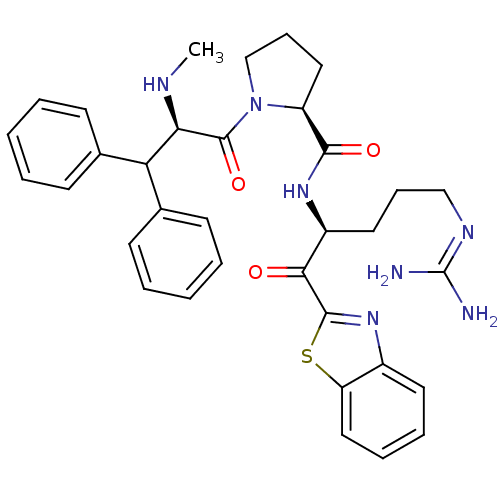
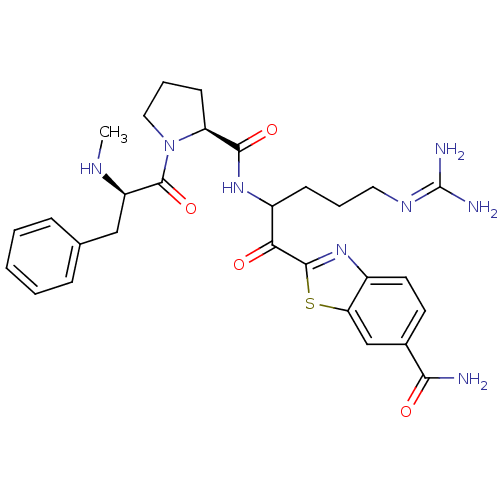
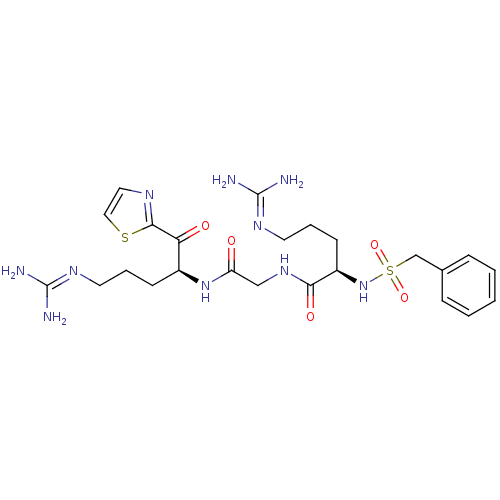
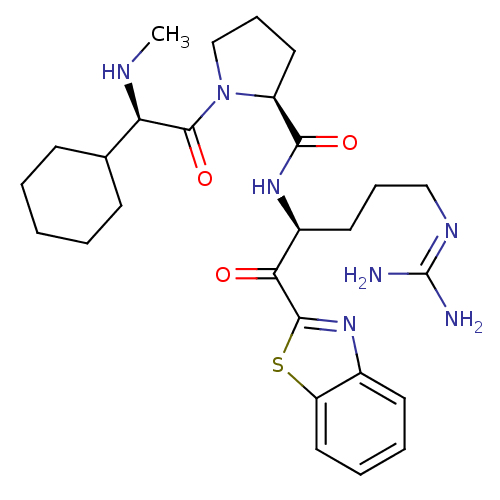
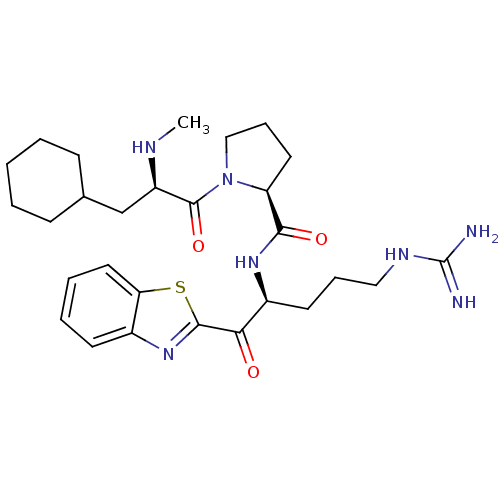
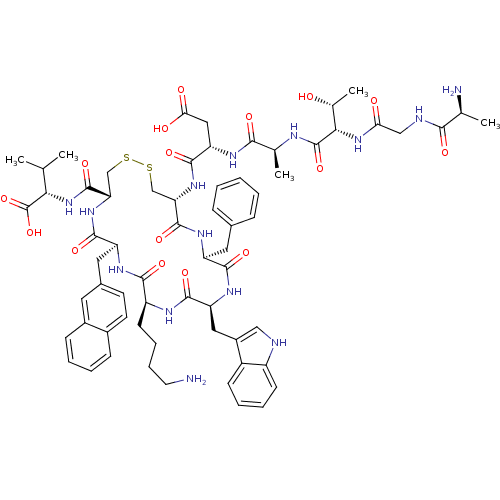
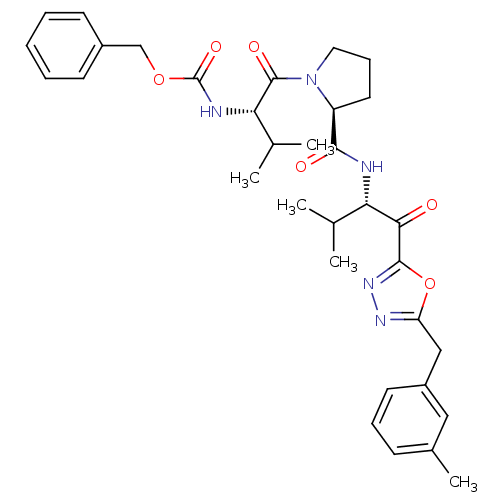
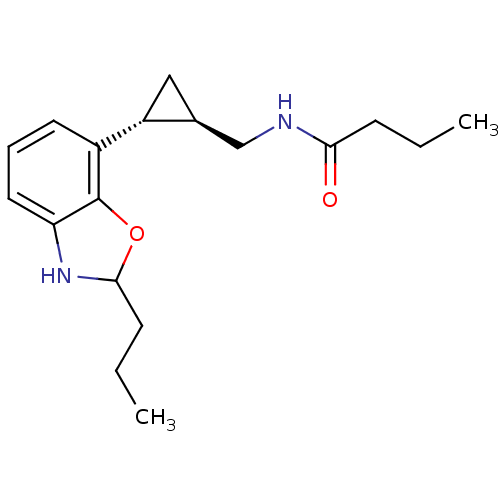
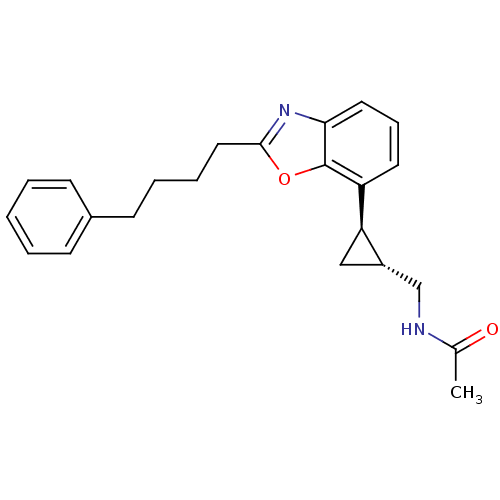
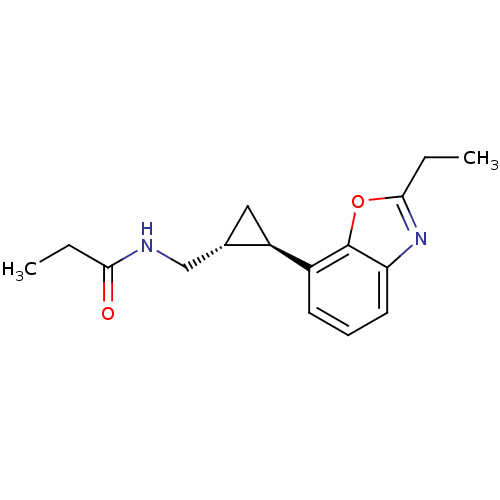
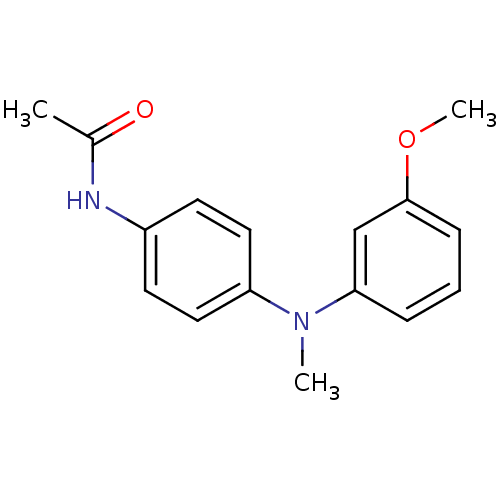
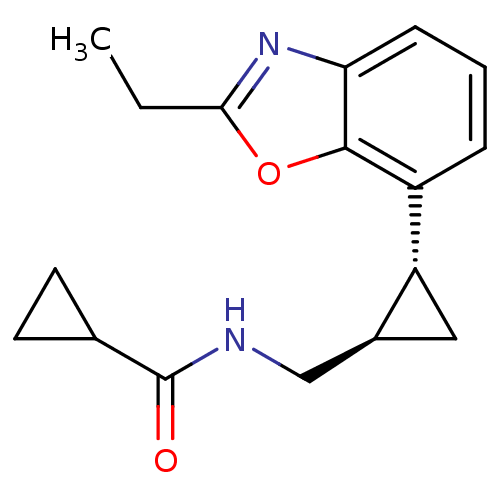
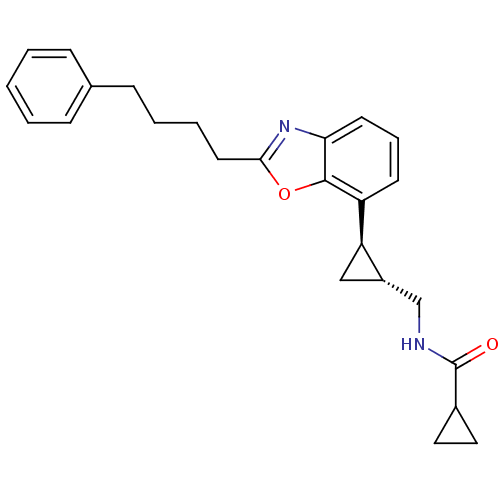
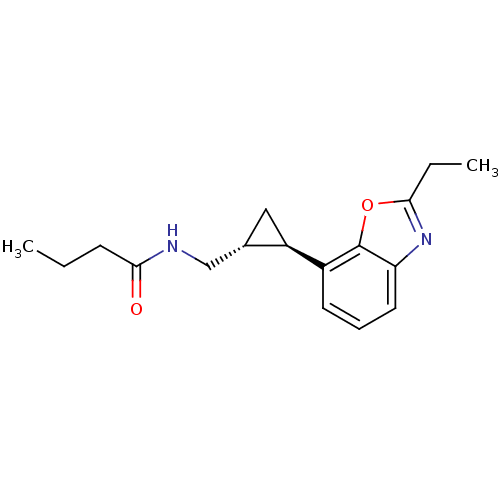
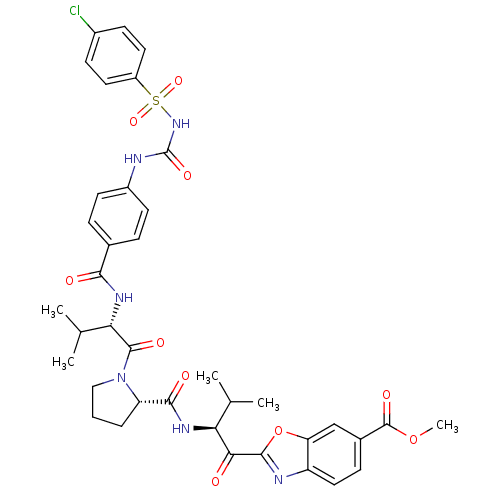
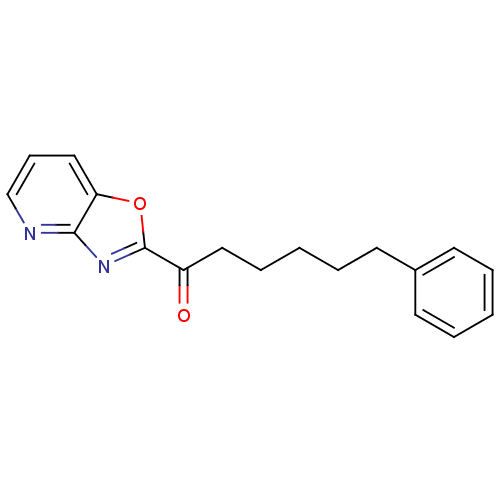
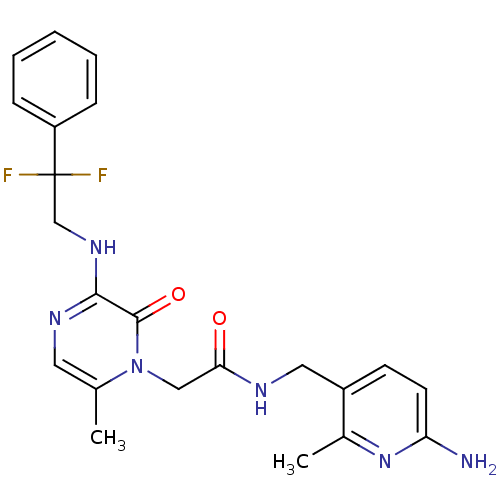
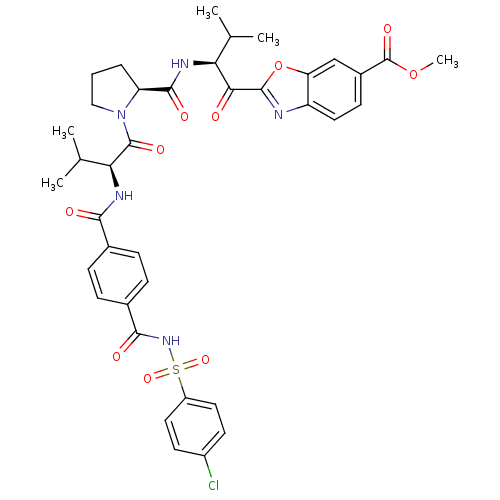
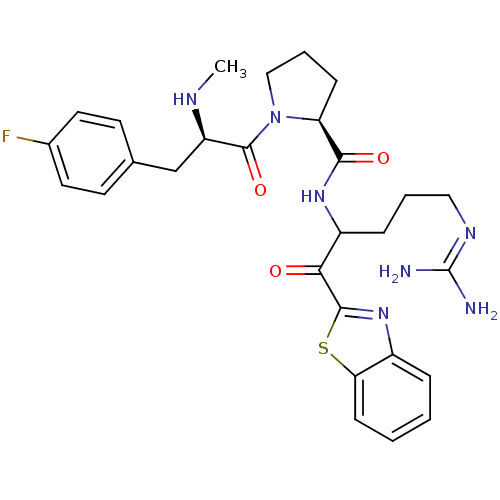
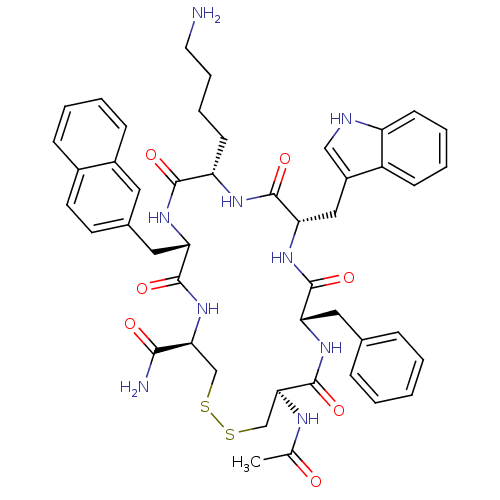
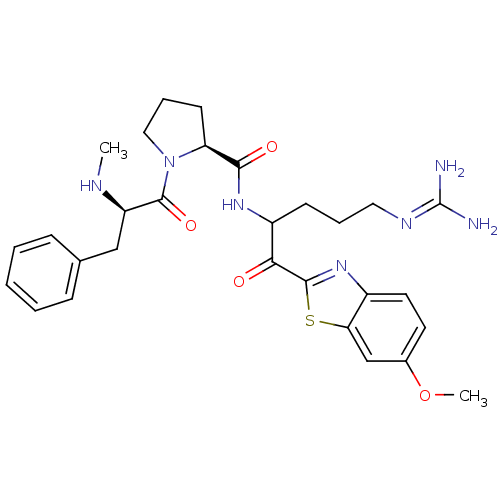
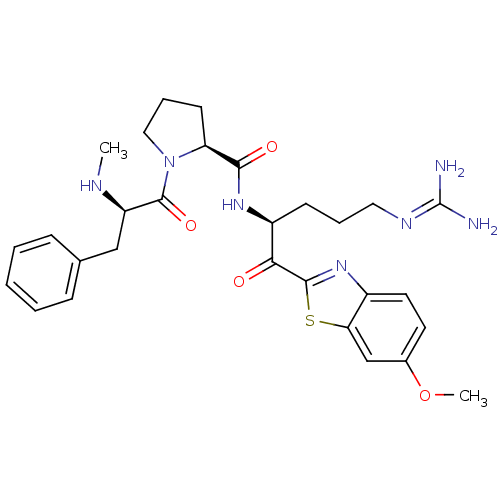
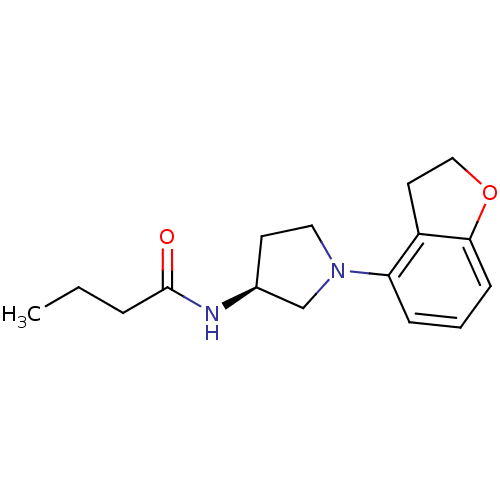
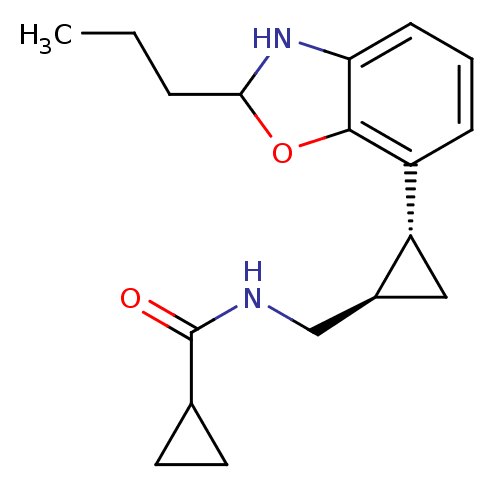
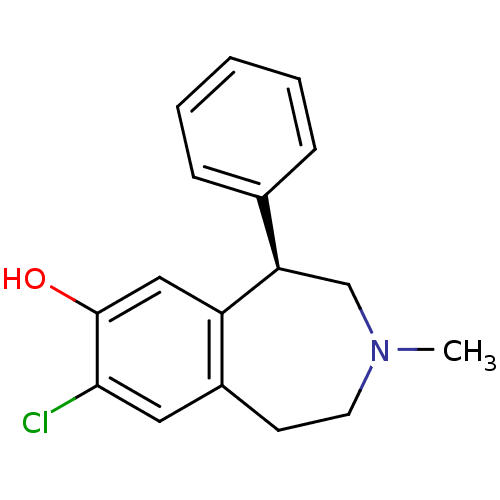
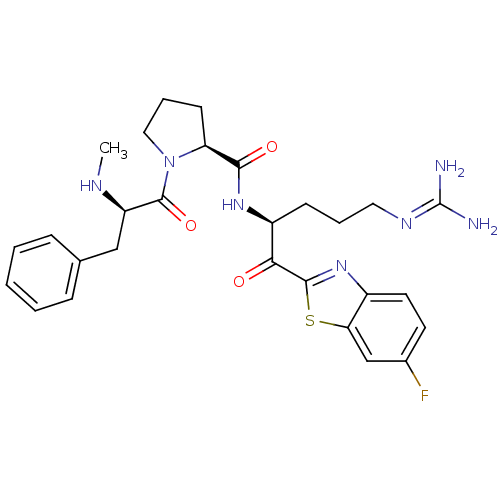
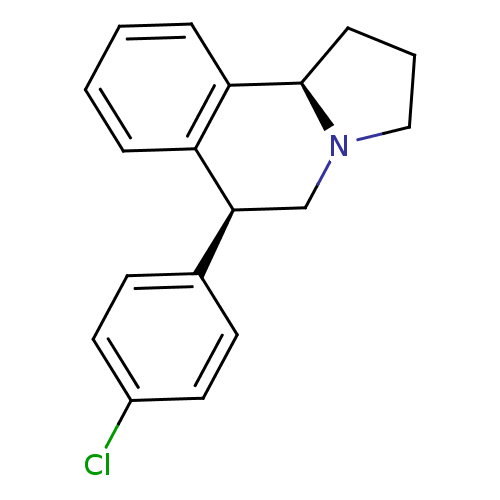
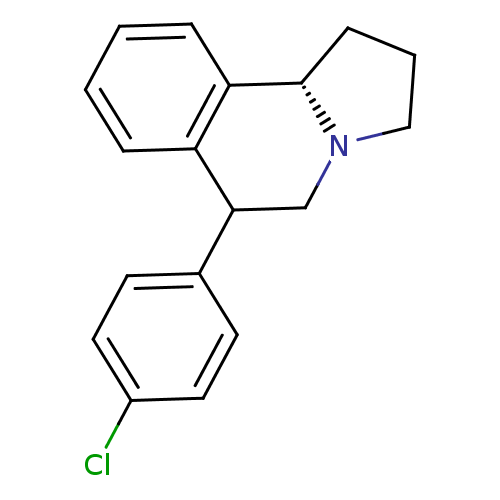
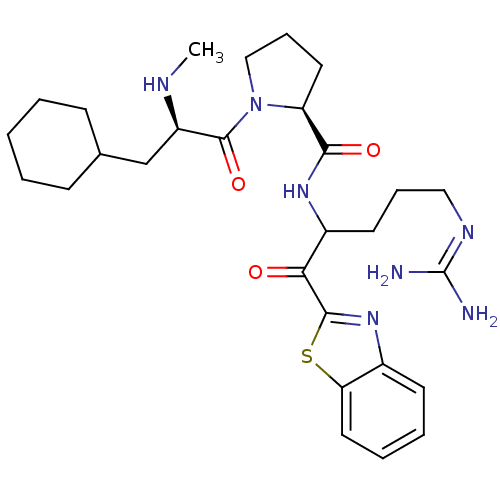
Affinity DataKi: 0.000650nM ΔG°: -72.4kJ/mole IC50: 4.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.000650nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.000650nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.00550nM ΔG°: -66.9kJ/mole IC50: 21nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.00700nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0140nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0180nM ΔG°: -63.8kJ/mole IC50: 5.30nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.0180nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0250nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0400nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1BMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding affinity towards human melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0500nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0600nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0700nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1BMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0700nMAssay Description:Binding affinity was determined against 5-hydroxytryptamine 1A receptor using [3H]WB-4101More data for this Ligand-Target Pair
Affinity DataKi: 0.0710nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1BMore data for this Ligand-Target Pair
TargetSerotonin 2 (5-HT2) receptor(RAT)
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0900nMAssay Description:Affinity for 5-hydroxytryptamine 2 receptorMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0940nMAssay Description:Inhibition of human recombinant FAAHMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Binding affinity towards human melatonin receptor type 1BMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -58.9kJ/mole IC50: 15nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Binding affinity towards human melatonin receptor type 1BMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1BMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1BMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Binding affinity towards Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM IC50: 7.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of norepinephrine (NE) into rat brain synaptosomesMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Binding affinity towards human melatonin receptor type 1BMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nM ΔG°: -57.9kJ/mole IC50: 48nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.180nMAssay Description:Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1AMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.180nMAssay Description:Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Binding affinity towards human melatonin receptor type 1BMore data for this Ligand-Target Pair