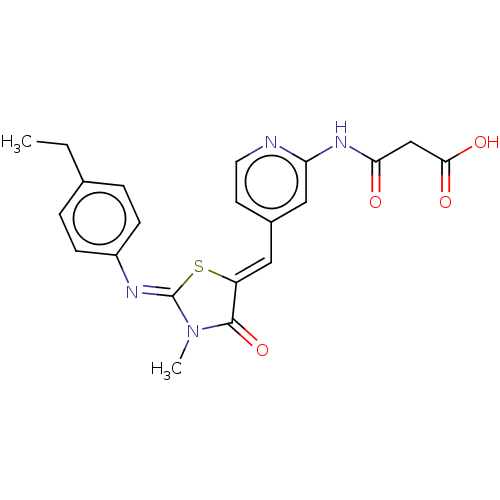
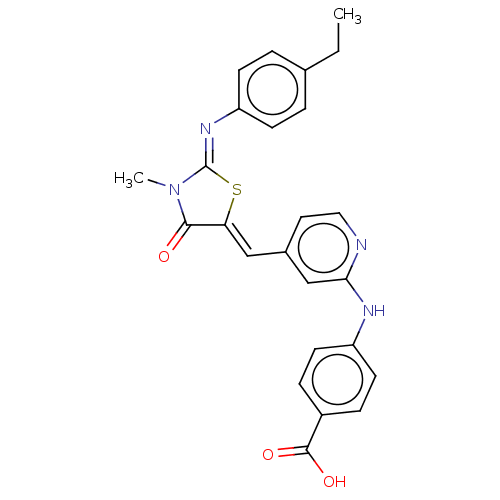
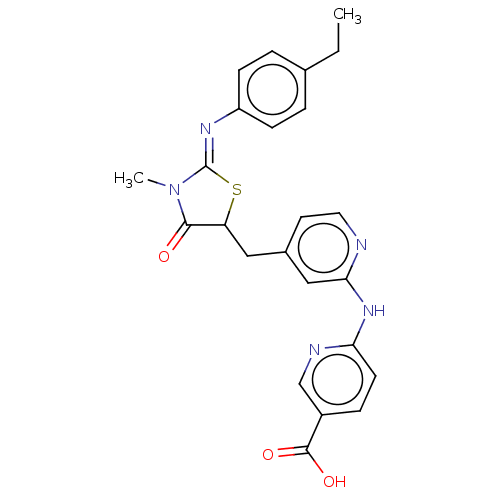
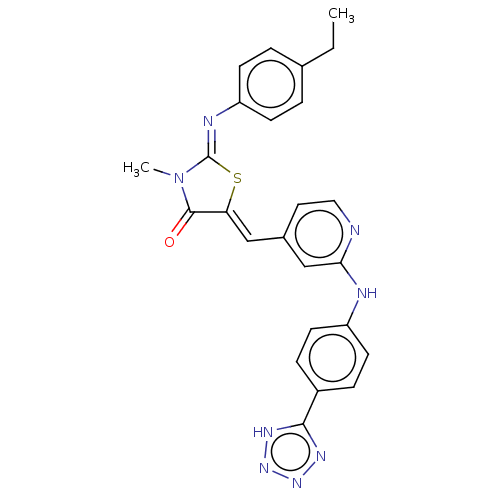
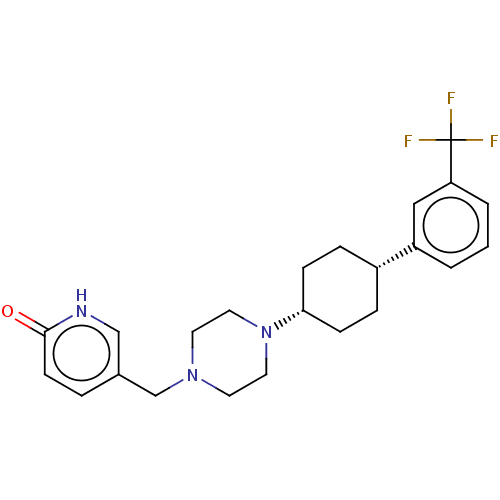
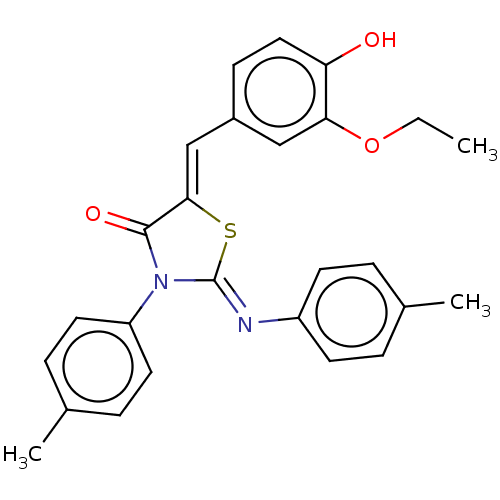
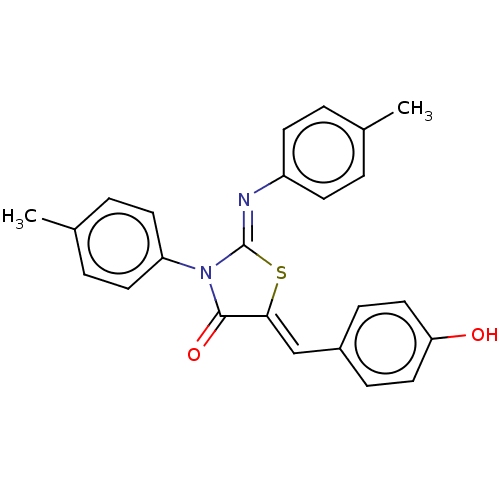
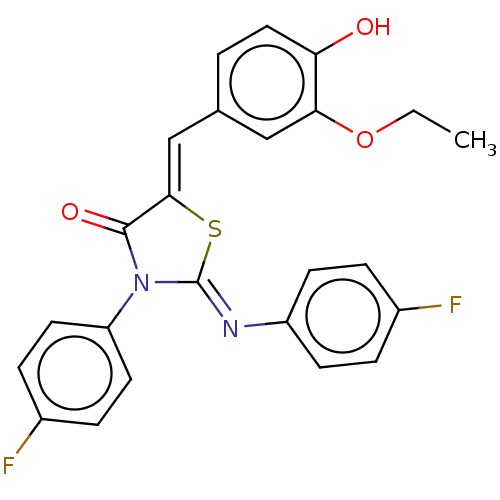
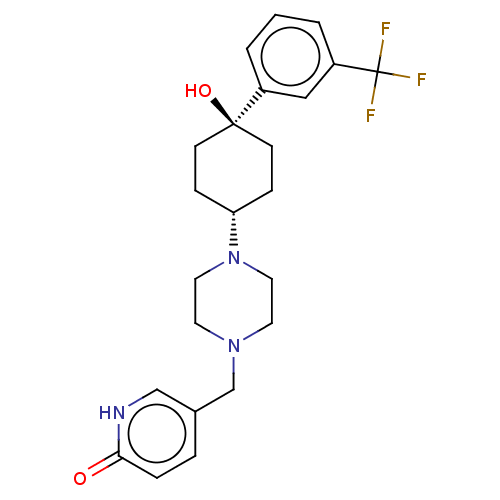
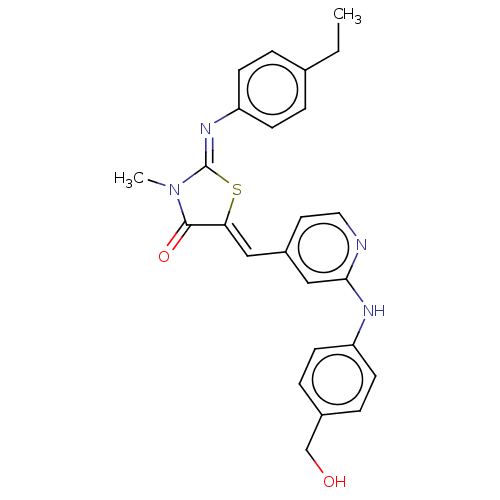
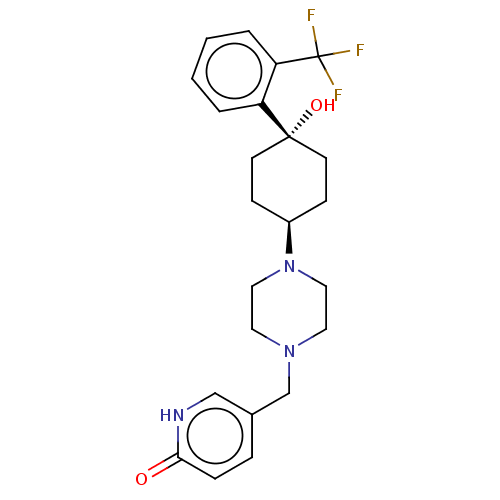
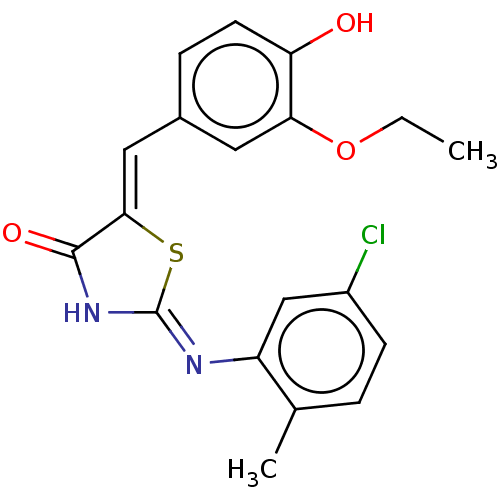
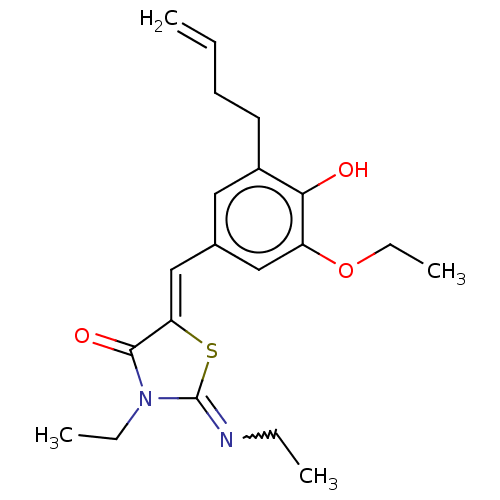
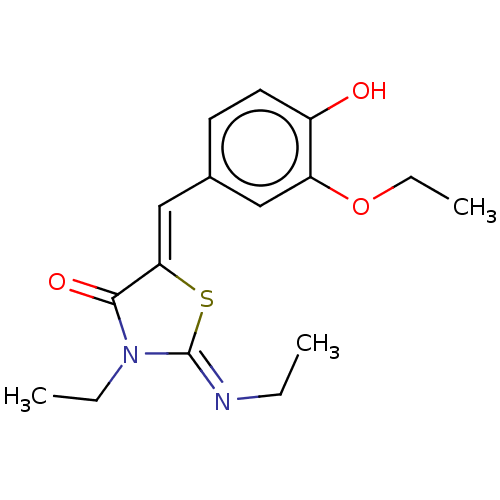
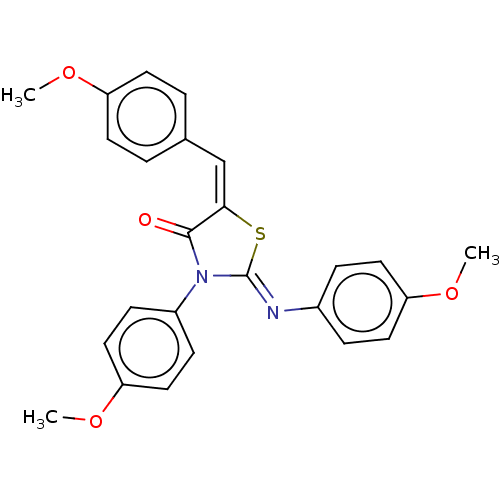
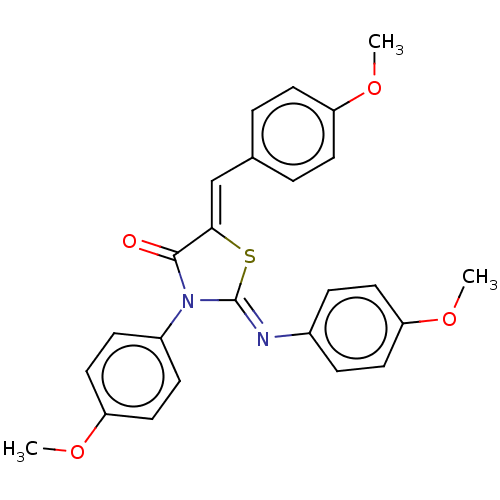
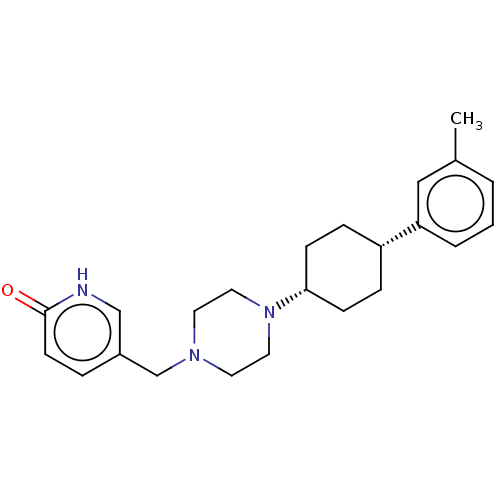
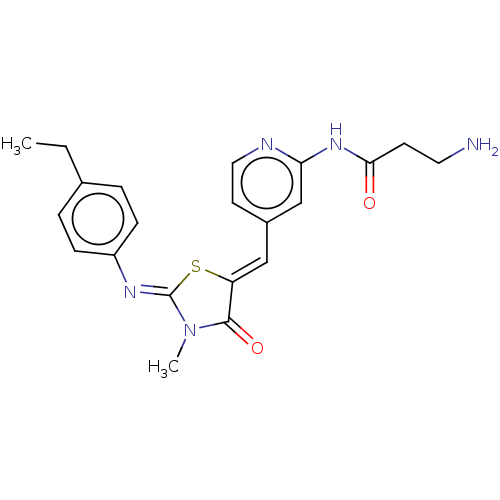
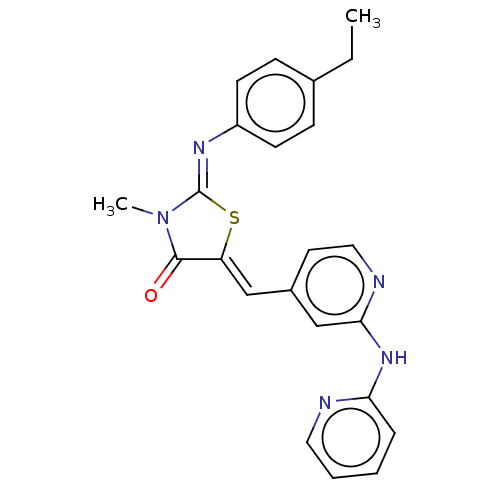
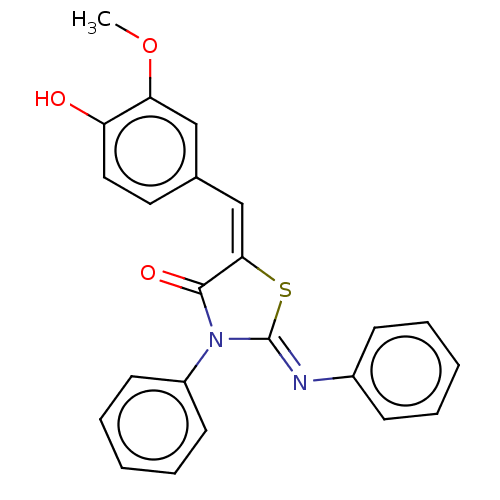
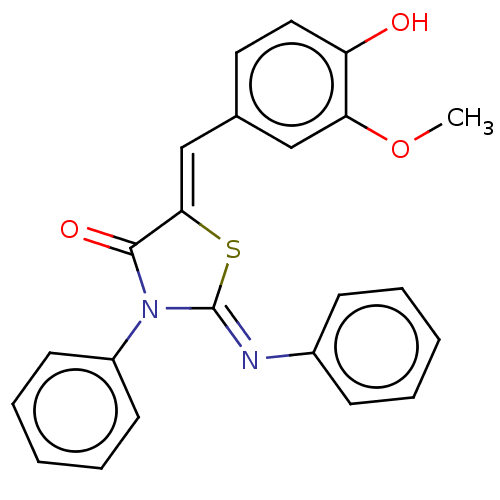
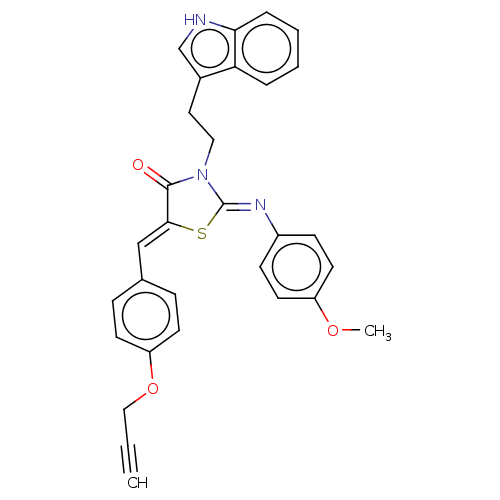
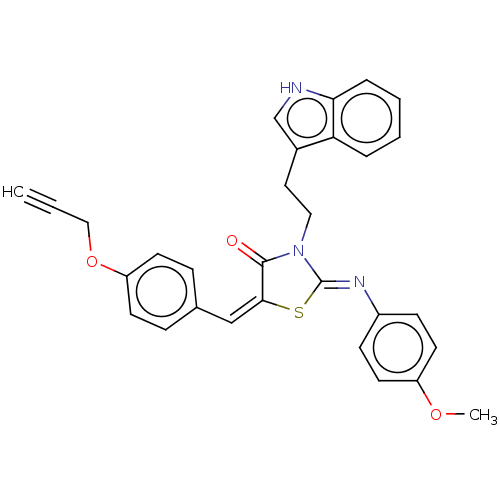
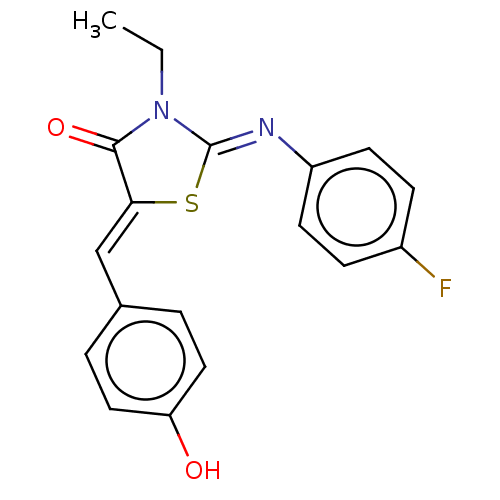
Affinity DataKi: 14nMAssay Description:Binding affinity to human alpha7 nAChRMore data for this Ligand-Target Pair
TargetNatural resistance-associated macrophage protein 2(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Competitive inhibition of human DMT1 expressed in HEK293T cells by measuring inhibition of radiolabeled-55Fe2+ uptake by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 5.20E+4nMAssay Description:Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0640nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
TargetAurora kinase B/Inner centromere protein(Homo sapiens (Human))
University Of Berne
Curated by ChEMBL
University Of Berne
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of Aurora B kinase in human HeLa Kyoto cells assessed as effect on distribution of phspho-histone H3 ser10 level incubated for 20 hrsMore data for this Ligand-Target Pair
TargetAurora kinase B/Inner centromere protein(Homo sapiens (Human))
University Of Berne
Curated by ChEMBL
University Of Berne
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of Aurora A kinase autophosphorylation at Thr288 in human HeLa Kyoto cells incubated for 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: >10nMAssay Description:Inhibition of Aurora B kinase in human HeLa Kyoto cells assessed as effect on distribution of phspho-histone H3 ser10 level incubated for 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of Aurora A kinase autophosphorylation at Thr288 in human HeLa Kyoto cells incubated for 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 64nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 138nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
TargetNatural resistance-associated macrophage protein 2(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human DMT1 expressed in Xenopus oocytes assessed as inhibition of channel currents at -50 mV holding potential by two-electrode voltage...More data for this Ligand-Target Pair
Affinity DataIC50: 145nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 145nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 148nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 148nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
TargetAurora kinase B/Inner centromere protein(Homo sapiens (Human))
University Of Berne
Curated by ChEMBL
University Of Berne
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 6(Homo sapiens (Human))
University Of Bern
Curated by ChEMBL
University Of Bern
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su...More data for this Ligand-Target Pair
Affinity DataIC50: 225nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 225nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 249nMAssay Description:Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 266nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 266nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair
Affinity DataIC50: 268nMAssay Description:Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ...More data for this Ligand-Target Pair

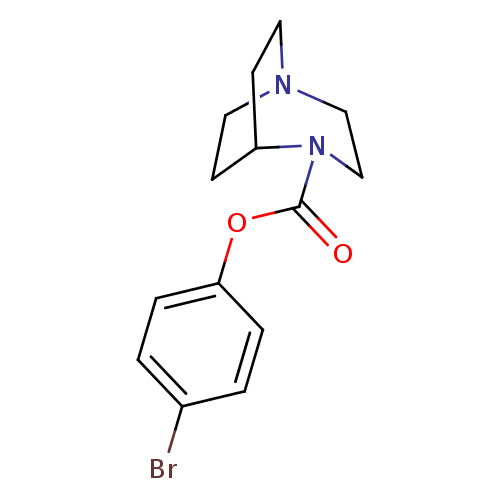

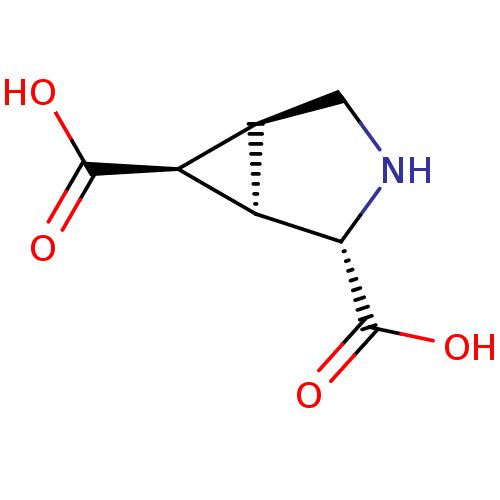
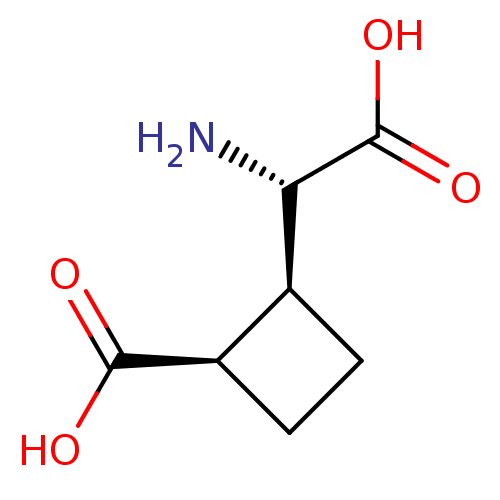


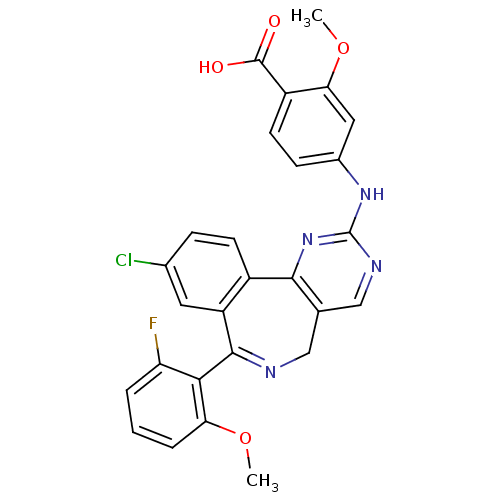
 3D Structure (crystal)
3D Structure (crystal)