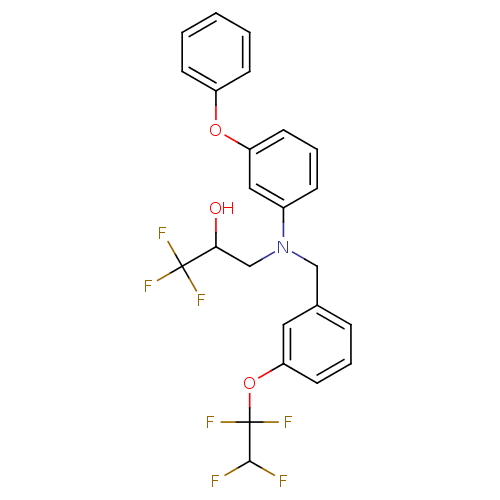
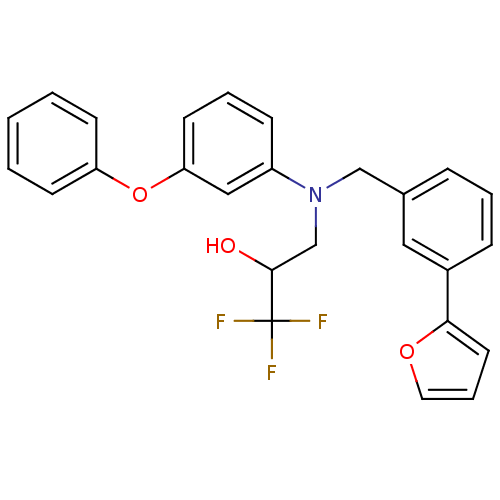
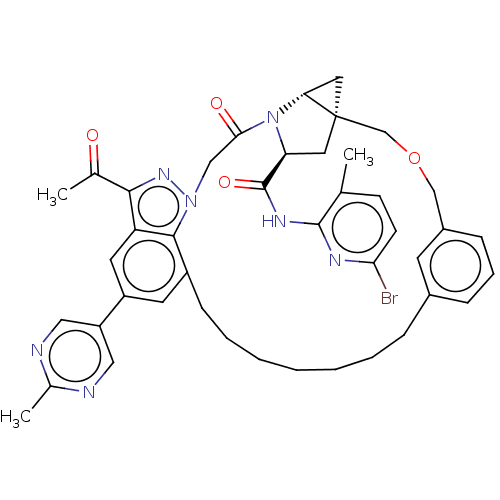
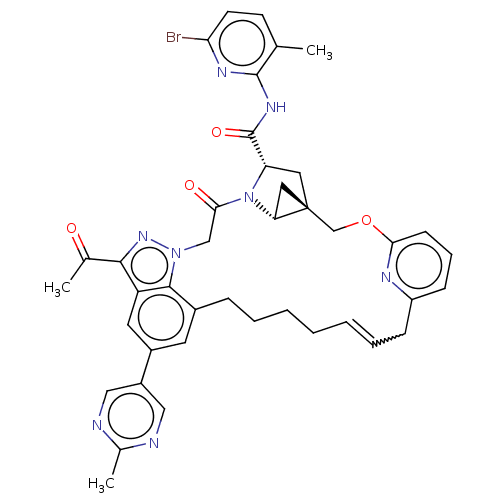
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 0.0420nMAssay Description:Inhibition of Thrombin (unknown origin)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibition of Thrombin (unknown origin)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 0.270nMAssay Description:Inhibition of Thrombin (unknown origin)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of Thrombin (unknown origin)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of Thrombin (unknown origin)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Mus musculus (Mouse))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]CP55940 from mouse brain membrane CB1 receptor after 60 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Inhibition of FKBP12 (unknown origin)-mediated rotamase activity by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Mus musculus (Mouse))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Displacement of [3H]CP55940 from mouse brain membrane CB1 receptor after 60 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 57nMAssay Description:Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 270nMAssay Description:Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 590nMAssay Description:Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ...More data for this Ligand-Target Pair
TargetTrypanothione reductase(Trypanosoma cruzi)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 2.40E+4nMAssay Description:Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assayMore data for this Ligand-Target Pair
TargetTrypanothione reductase(Trypanosoma cruzi)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assayMore data for this Ligand-Target Pair
TargetTrypanothione reductase(Trypanosoma cruzi)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 8.40E+4nMAssay Description:Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assayMore data for this Ligand-Target Pair
Target4-aminobutyrate aminotransferase, mitochondrial(Sus scrofa)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 6.30E+6nMAssay Description:Competitive inhibition of pig brain GABA aminotransferase by Dixon/Cornish-Bowden plot analysis in presence of GABAMore data for this Ligand-Target Pair
Target4-aminobutyrate aminotransferase, mitochondrial(Sus scrofa)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10E+7nMAssay Description:Competitive inhibition of pig brain GABA aminotransferase by Dixon/Cornish-Bowden plot analysis in presence of GABAMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by...More data for this Ligand-Target Pair
Affinity DataIC50: 2.05nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.32nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.87nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.42nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.13nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.25nMpH: 7.5 T: 2°CAssay Description:An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of DPP-4 (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 52nMAssay Description:Inhibition of recombinant human Tissue factor/factor 7a using N-methylsulfonyl-D-phe-gly-arg-p-nitroaniline as substrate assessed as release of p-nit...More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 112nMAssay Description:Antagonist activity at human ETA receptor expressed in CHO cells assessed as inhibition of ET-1-induced Ca2+ efflux from endoplasmic reticulum into c...More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Antagonist activity at human ETA receptor expressed in CHO cells assessed as inhibition of ET-1-induced Ca2+ efflux from endoplasmic reticulum into c...More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of recombinant human CETP in buffer assessed as transfer of [3H]-cholesteryl ester from HDL donor particles to LDL acceptor particlesMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by...More data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of recombinant human Tissue factor/factor 7a using N-methylsulfonyl-D-phe-gly-arg-p-nitroaniline as substrate assessed as release of p-nit...More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibition of recombinant human CETP in buffer assessed as transfer of [3H]-cholesteryl ester from HDL donor particles to LDL acceptor particlesMore data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce...More data for this Ligand-Target Pair


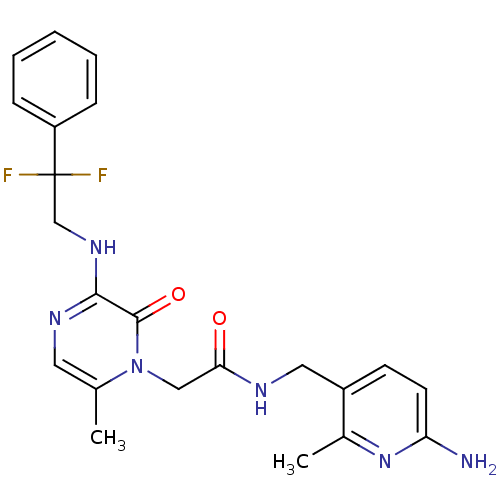
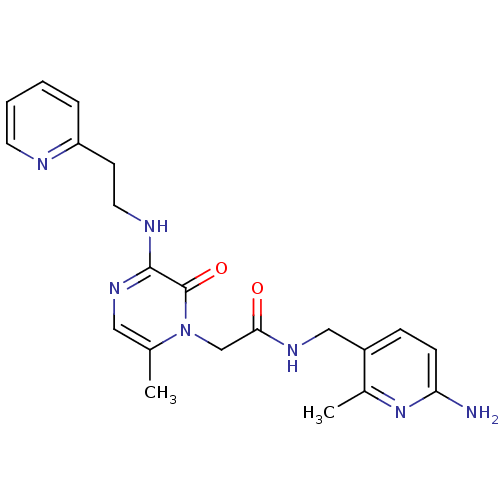



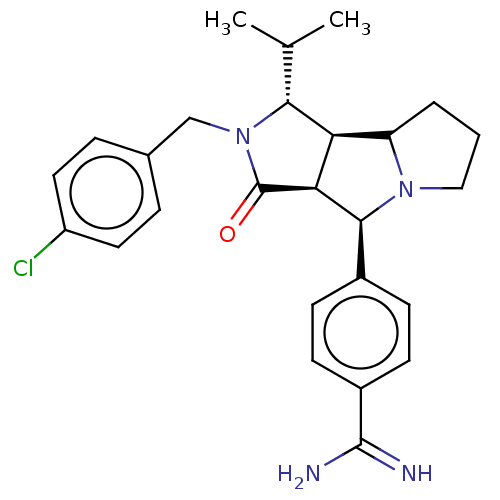


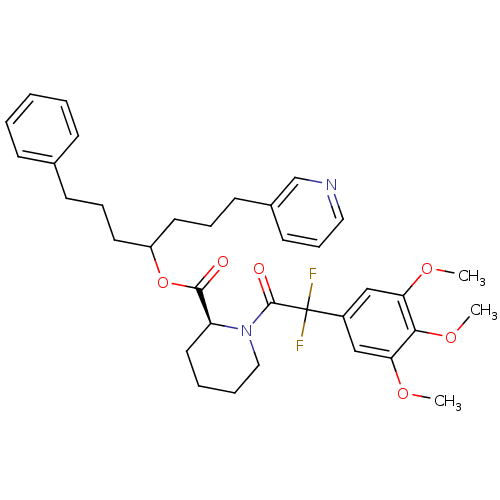











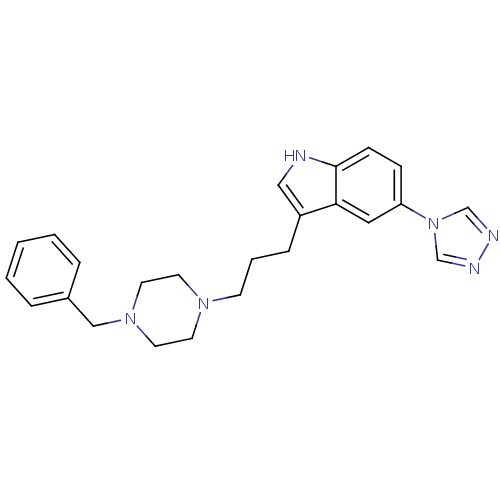
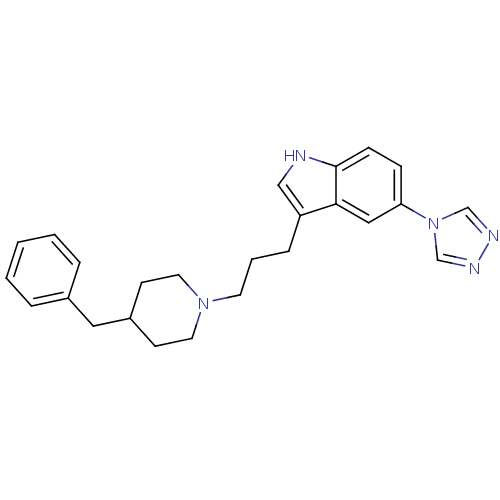


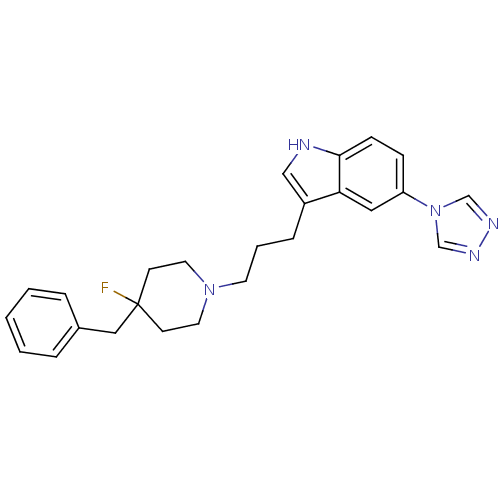

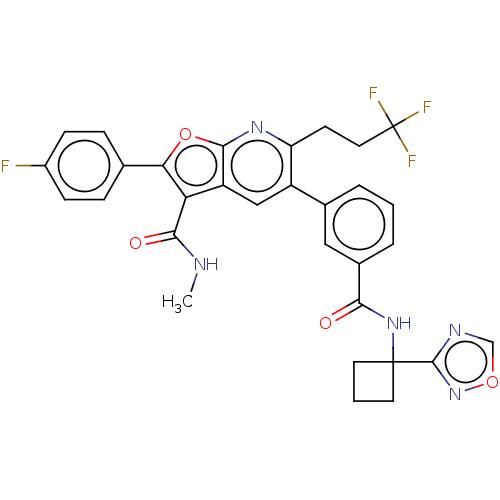

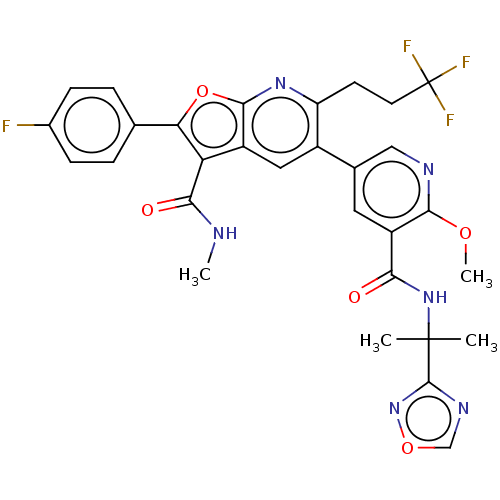
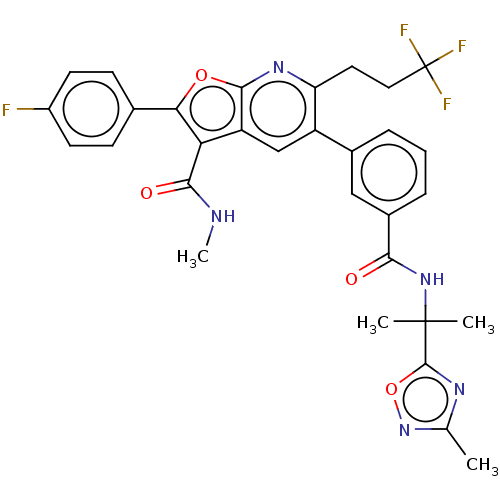
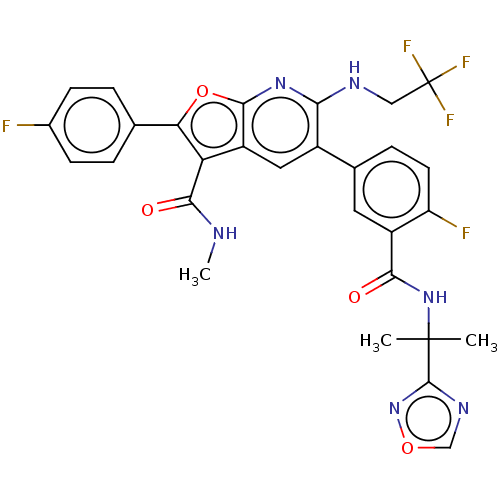
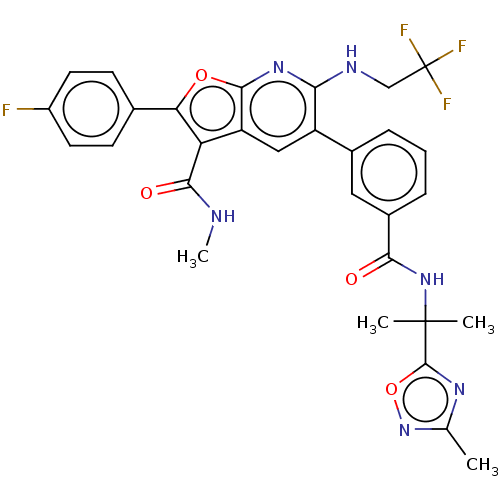
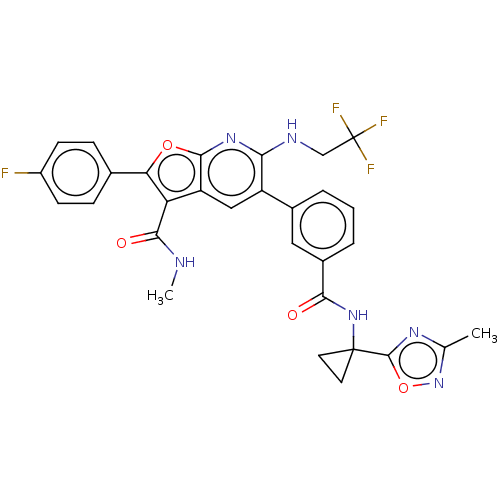
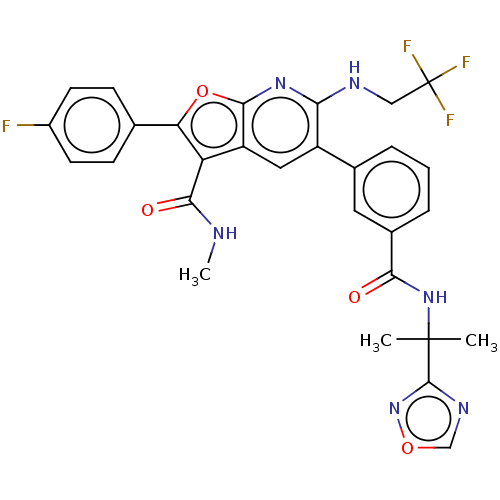
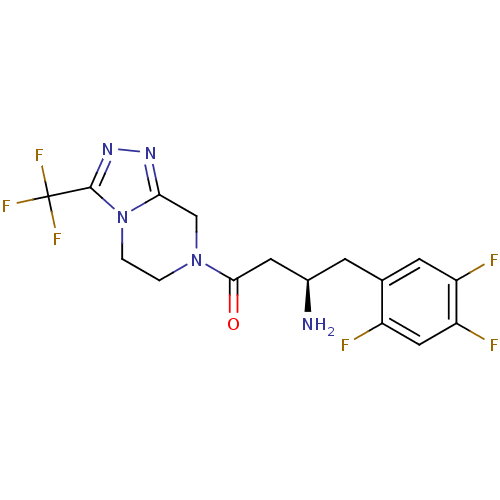
 3D Structure (crystal)
3D Structure (crystal)