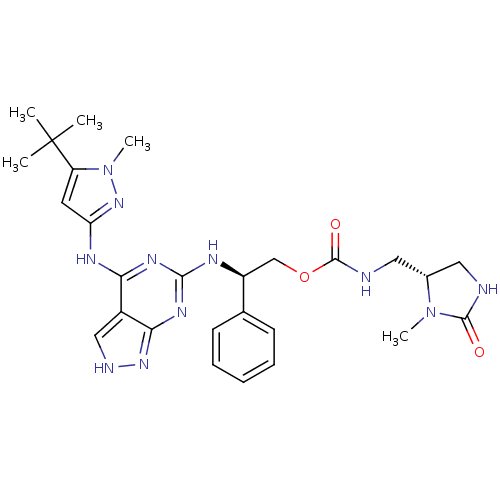
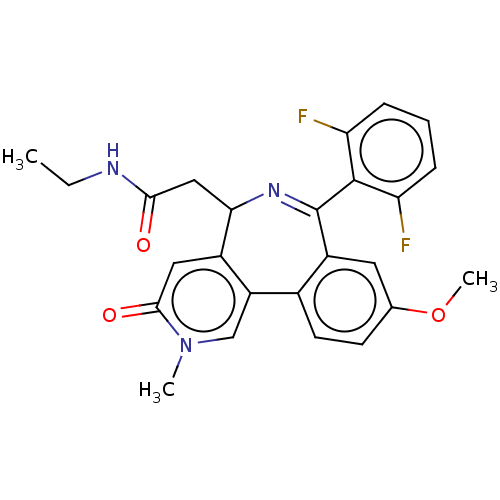
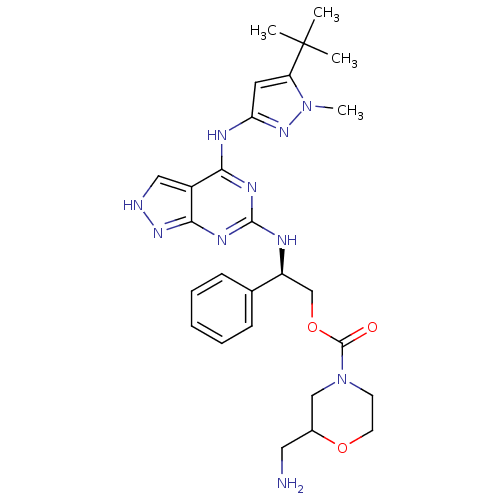
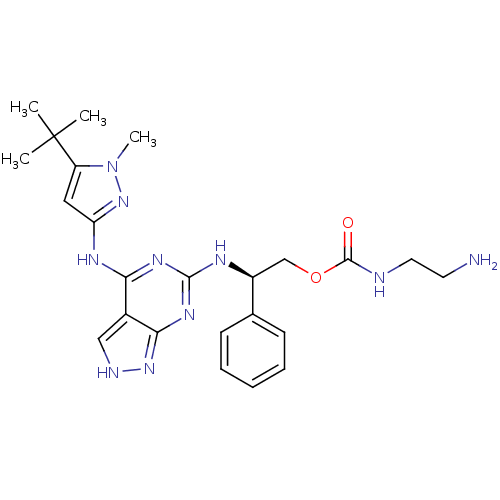
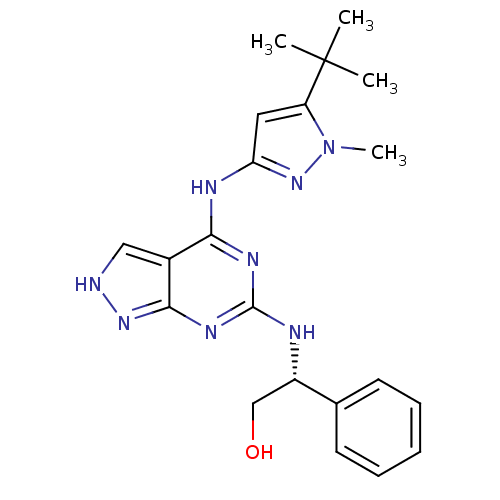
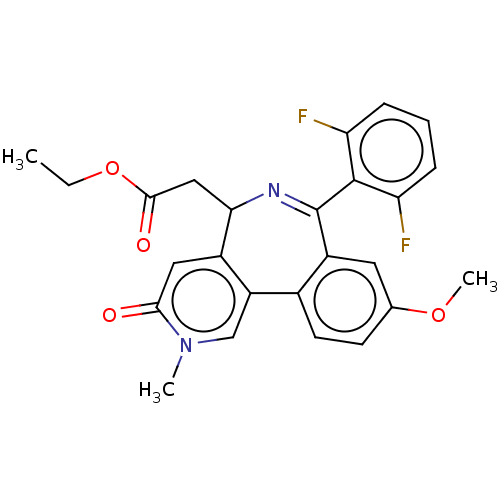
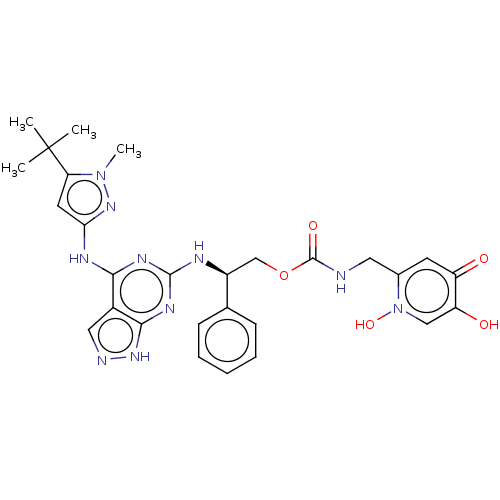
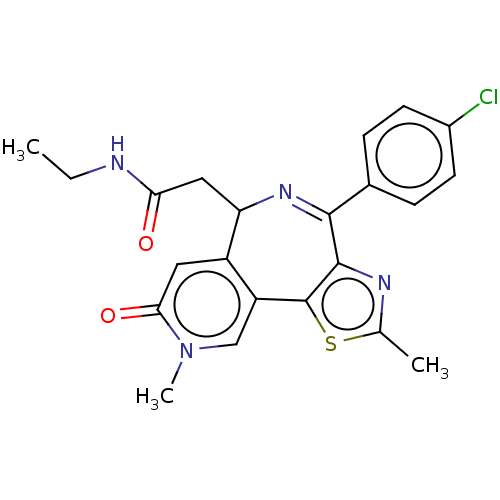
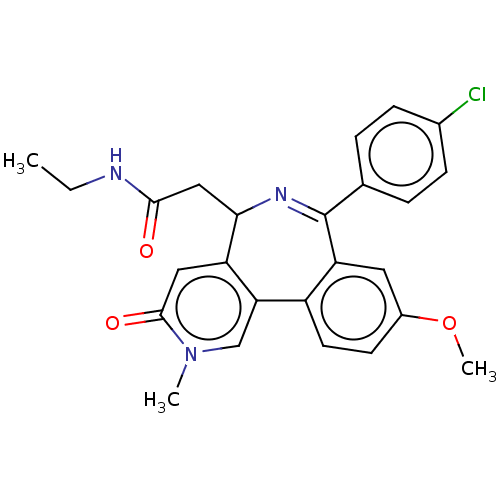
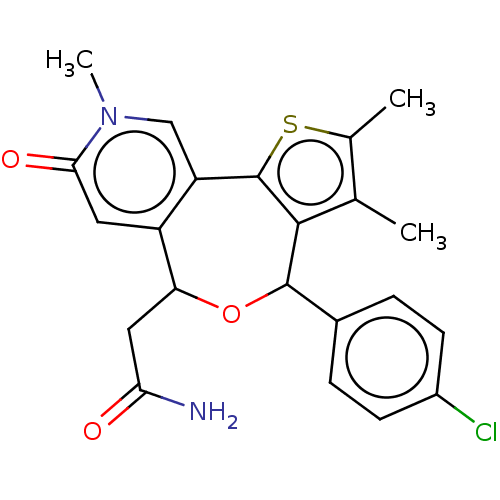
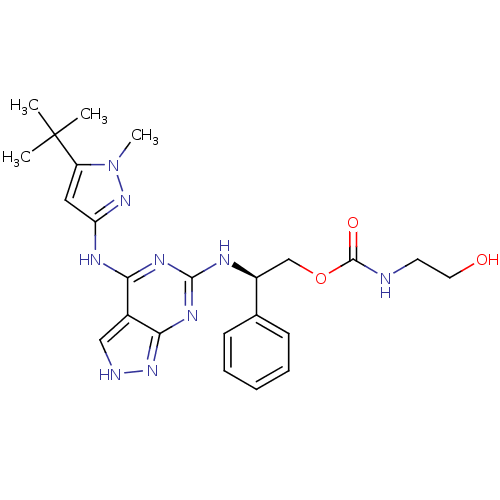
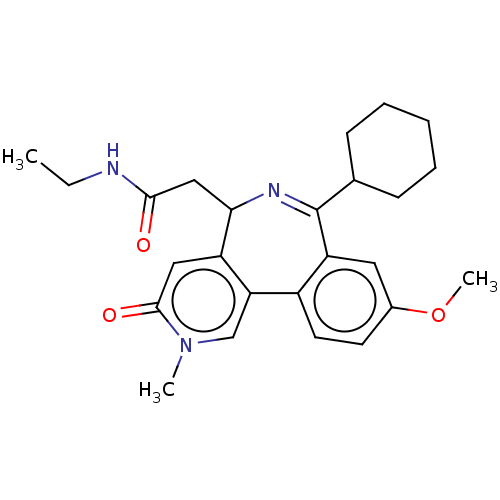
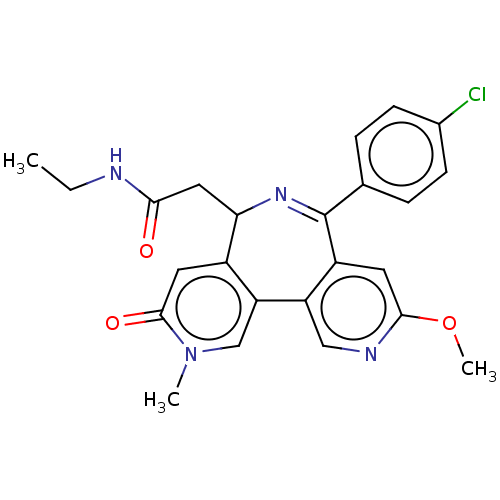
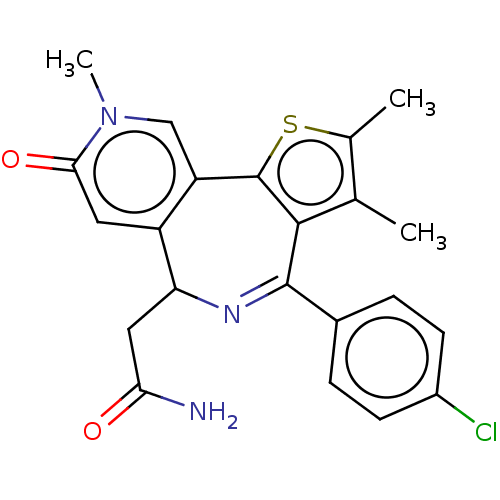
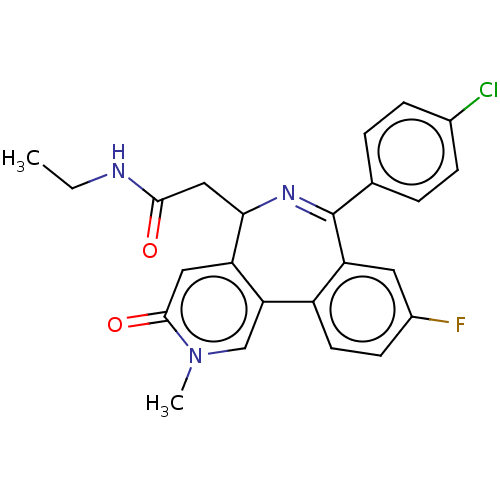
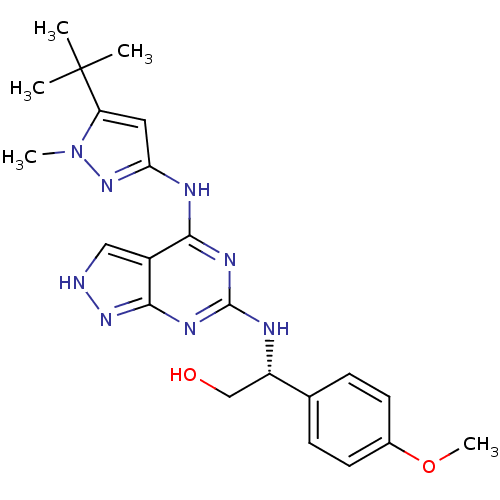
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
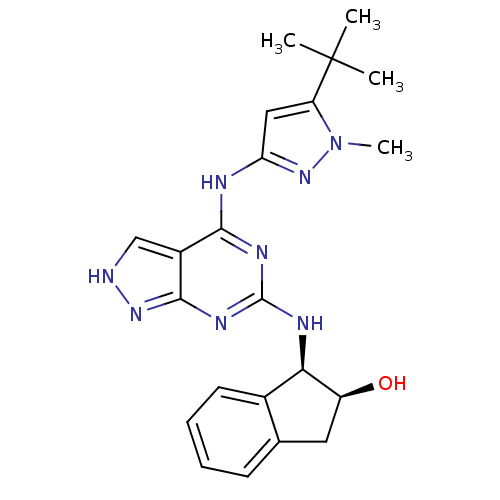
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
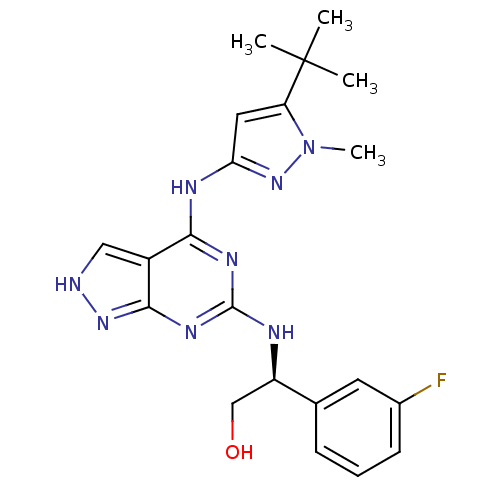
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.700nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1.20nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1.30nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 2nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair