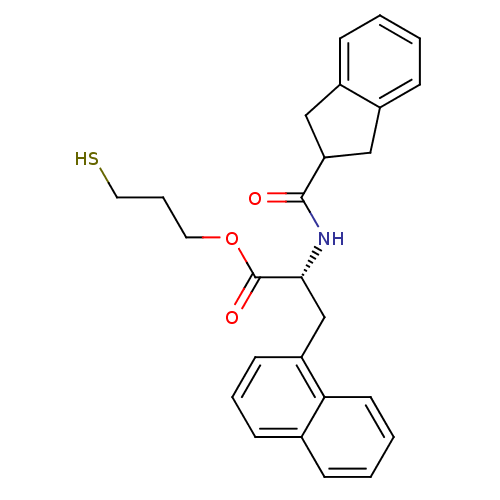
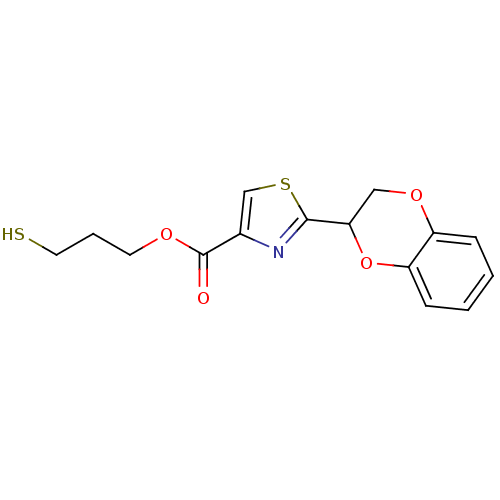
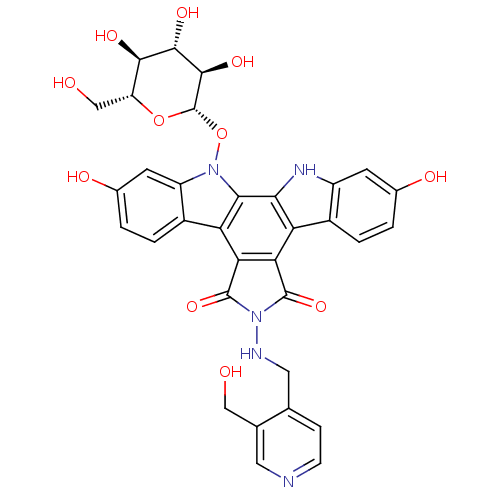
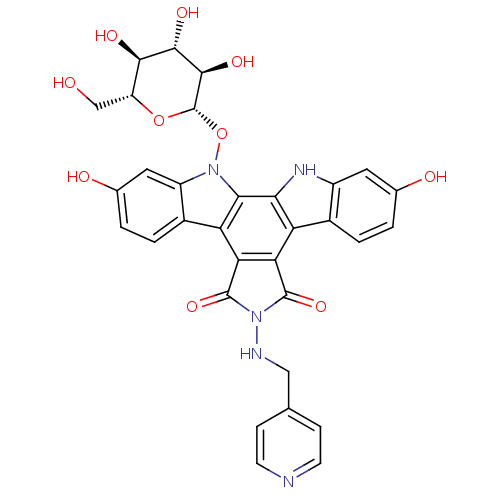
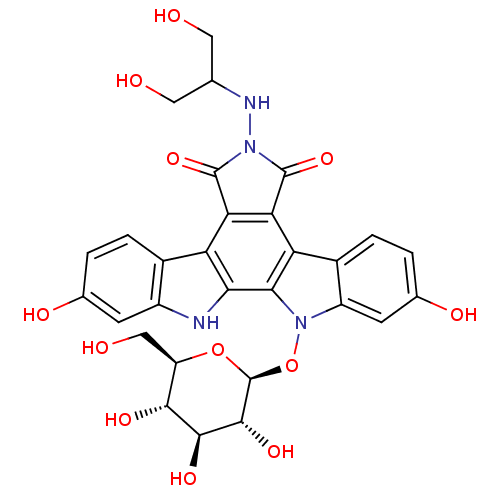
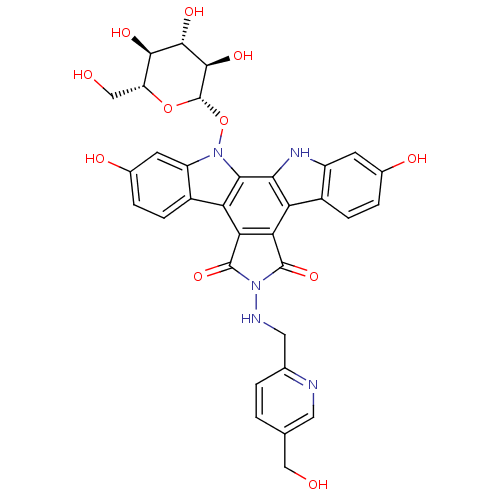
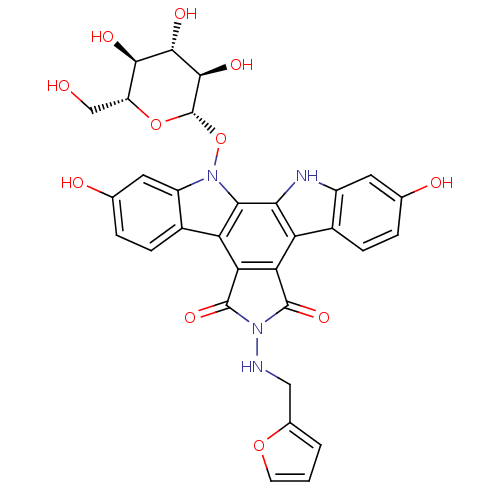
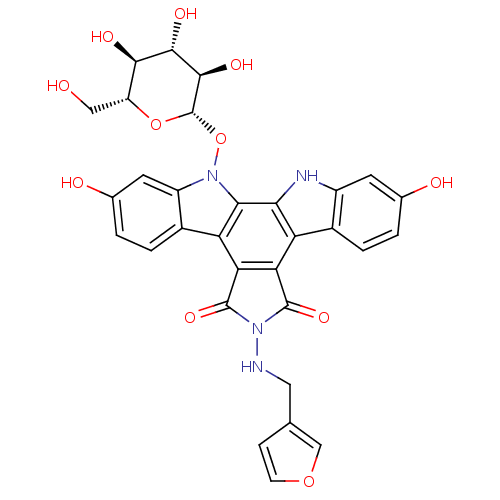
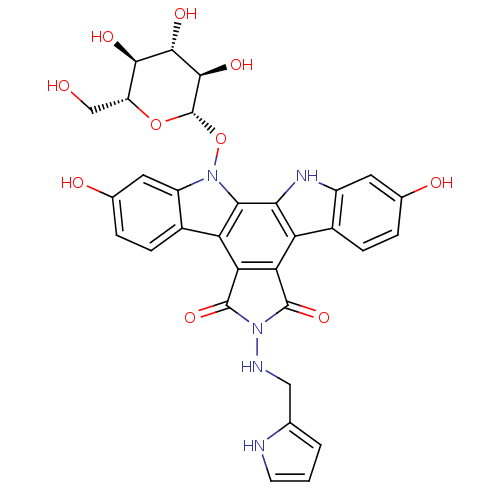
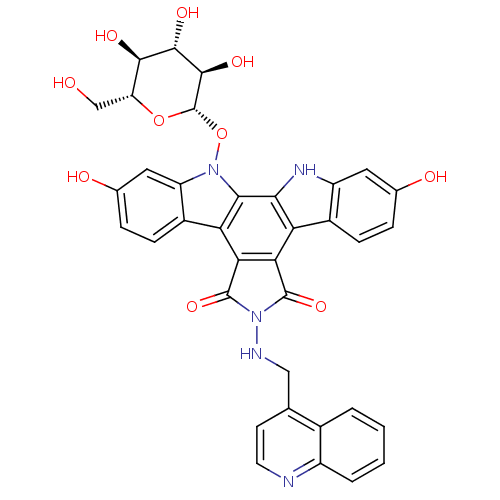
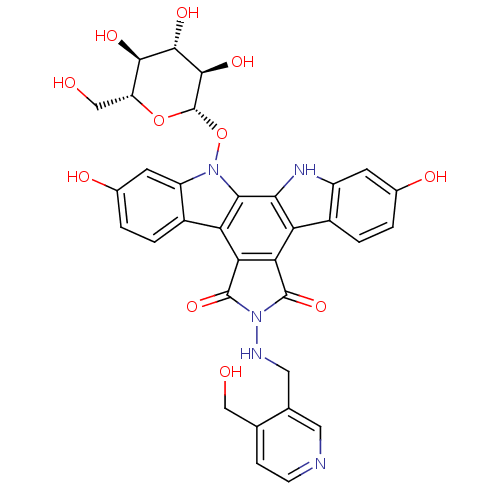
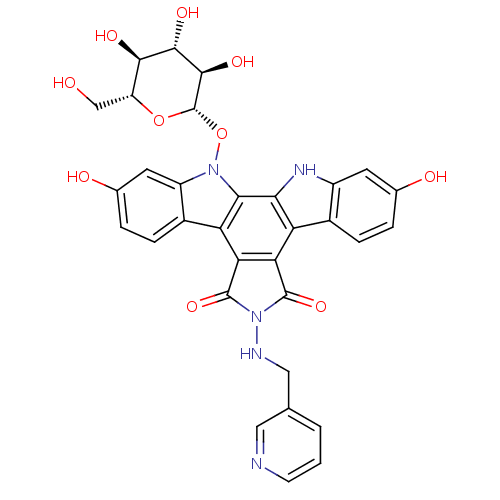
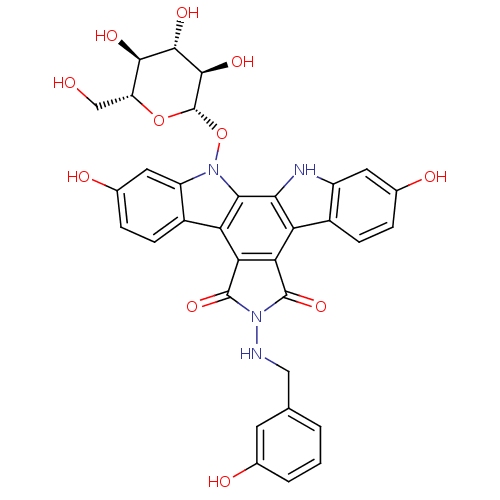
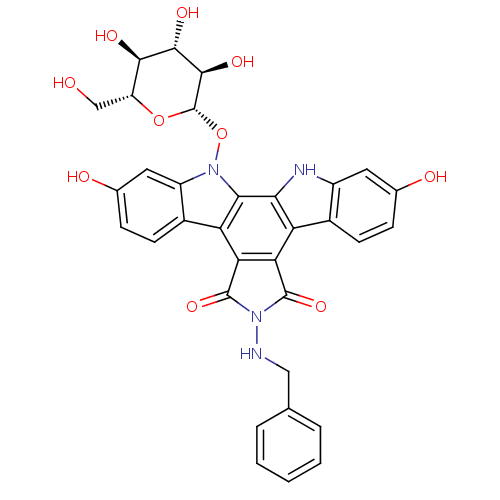
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Tested for the inhibition of Candida GGTase I in Candida albicansMore data for this Ligand-Target Pair
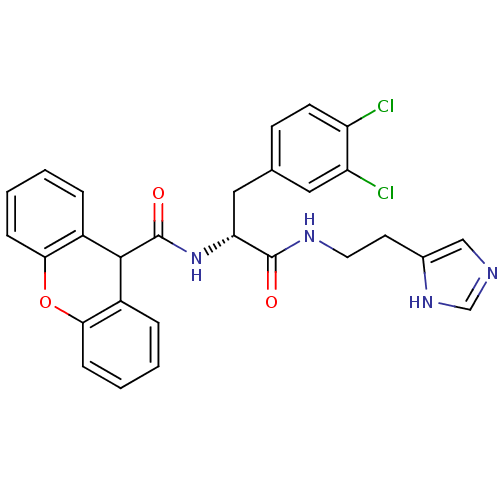
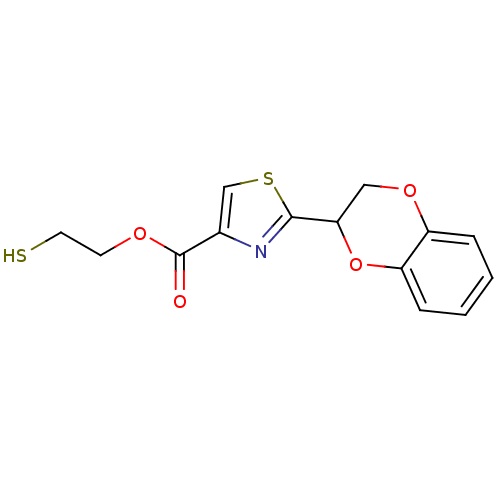
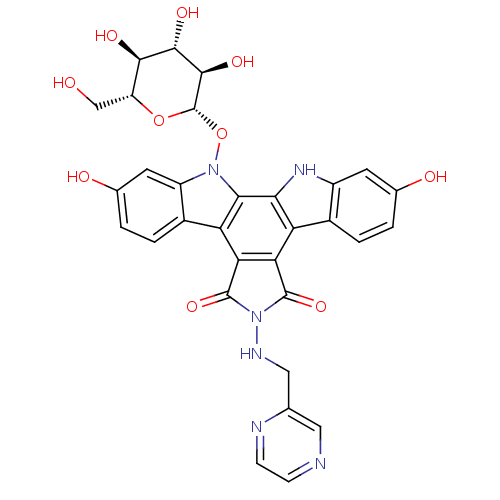
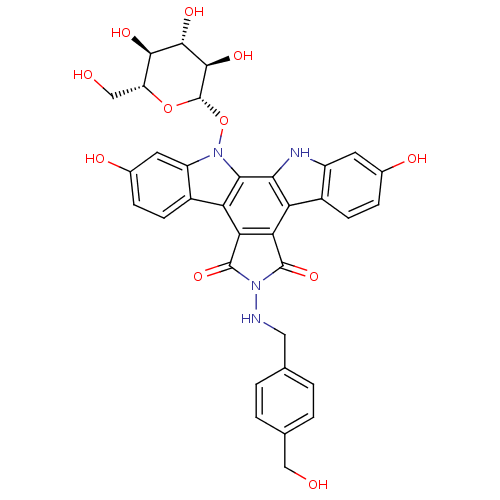
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of Candida geranylgeranyl transferase I at 3 uMMore data for this Ligand-Target Pair
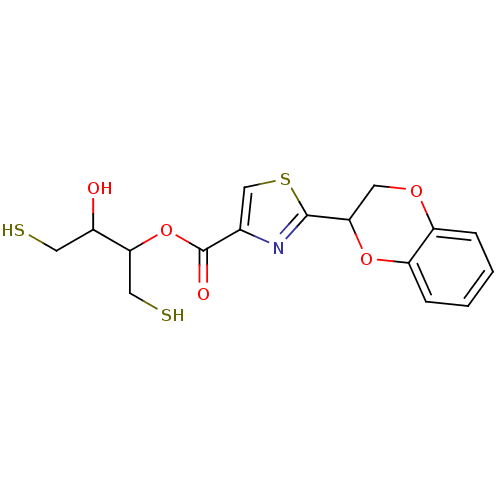
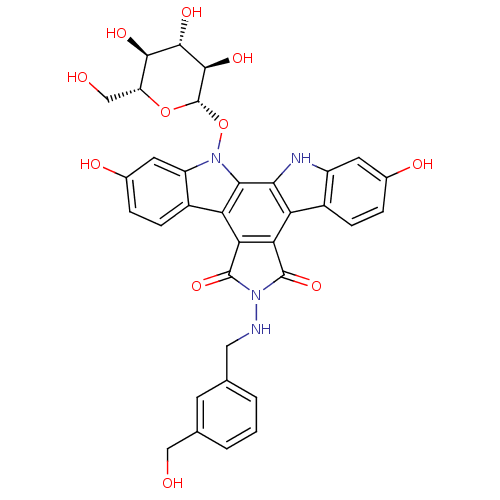
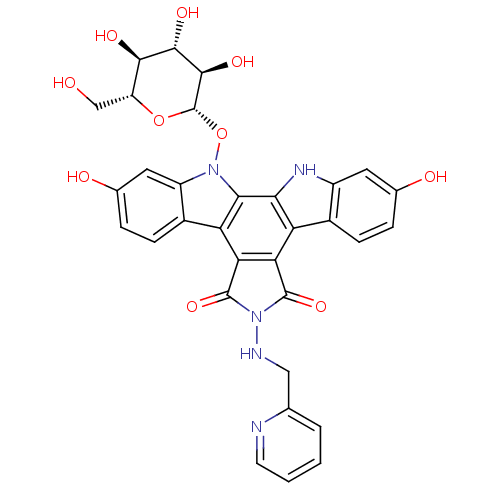
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Tested for the inhibition of Candida GGTase I in Candida albicansMore data for this Ligand-Target Pair
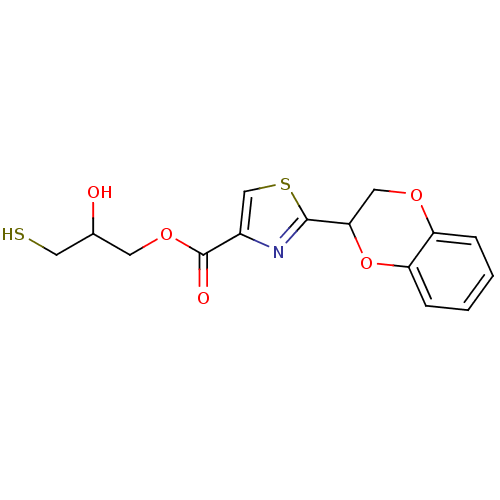
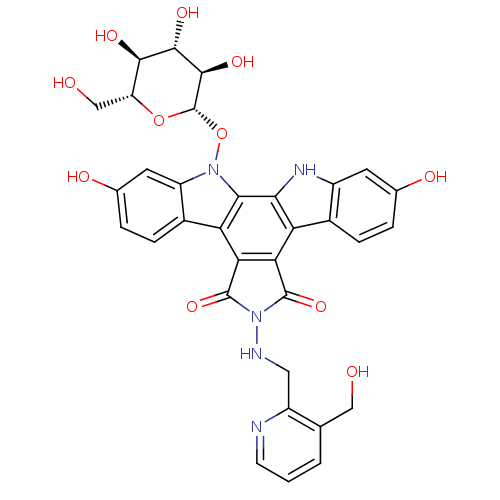
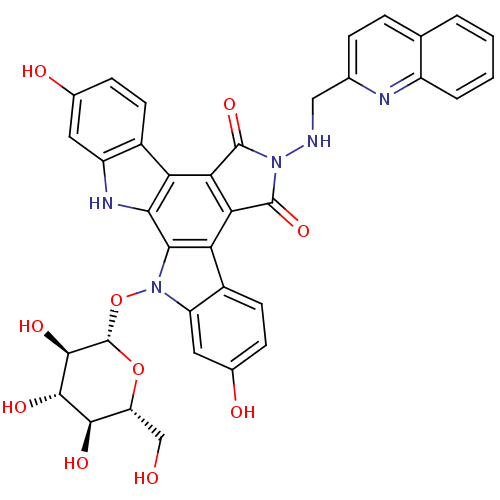
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Tested for the inhibition of Candida GGTase I in Candida albicansMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Tested for the inhibition of Candida GGTase I in Candida albicansMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of Candida geranylgeranyl transferase I at 3 uMMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of Candida geranylgeranyl transferase IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 780nMAssay Description:Tested for the inhibition of Candida GGTase I in Candida albicansMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of Candida geranylgeranyl transferase I at 3 uMMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human geranylgeranyl transferase IMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
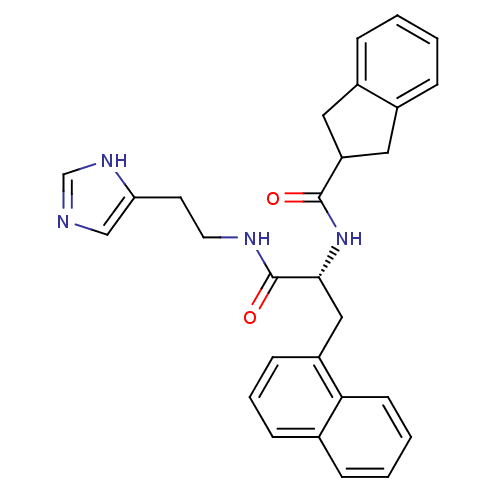
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human farnesyl transferaseMore data for this Ligand-Target Pair
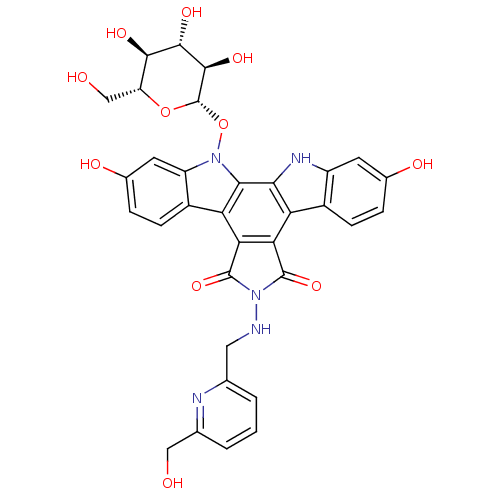
Affinity DataEC50: 120nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 180nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 210nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 220nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 300nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 420nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 470nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 600nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 800nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 800nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+3nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80E+3nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 6.00E+3nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 6.50E+3nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Inhibition of DNA topoisomerase 1 in mouse P388/S cells assessed as formation of topoisomerase 1-DNA complex by protinase K/SDS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 27nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated DNA cleavage assessed as relaxation of supercoiled pBR322 plasmid DNA after 15 mins by densitometerMore data for this Ligand-Target Pair