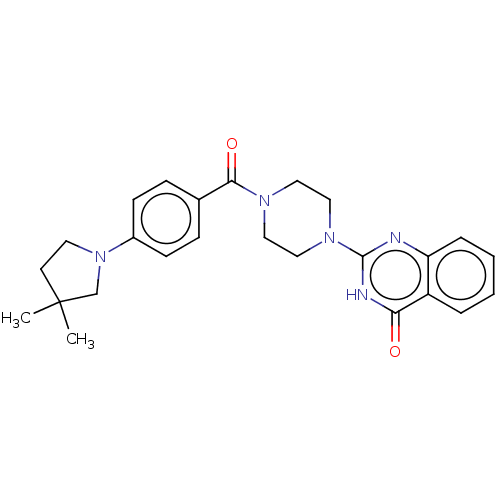
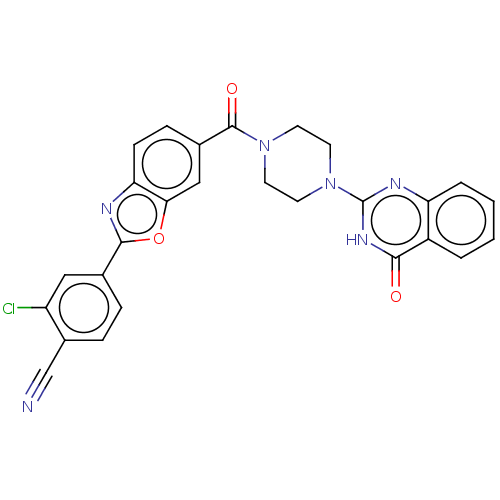
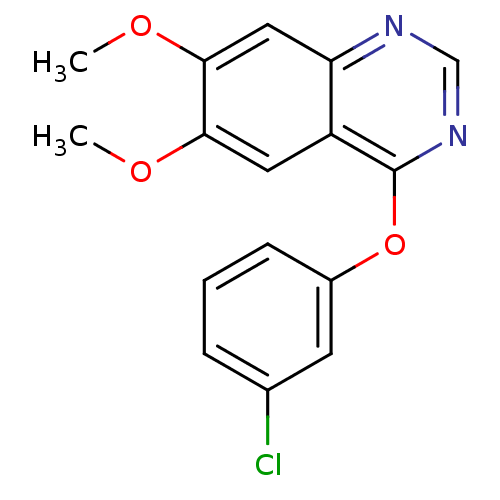
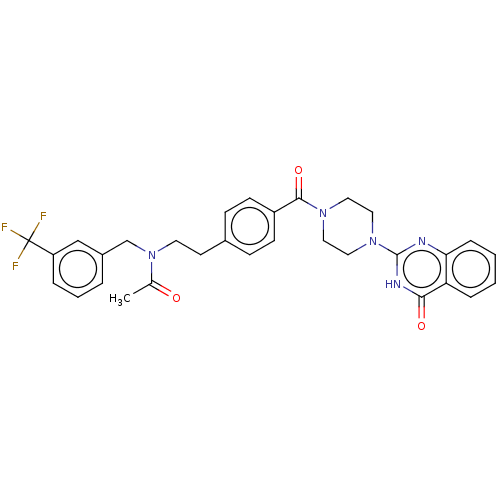
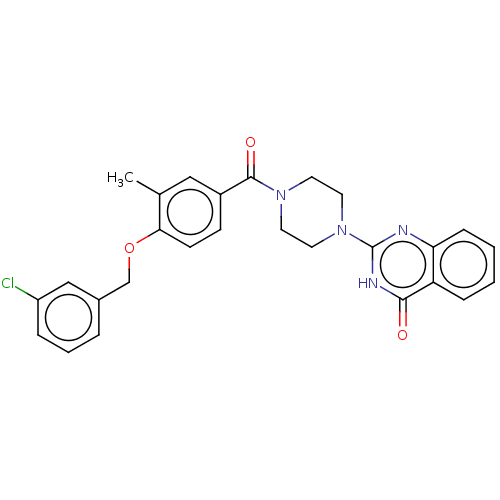
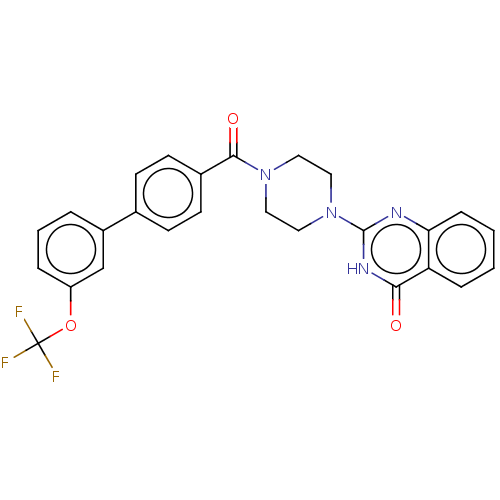
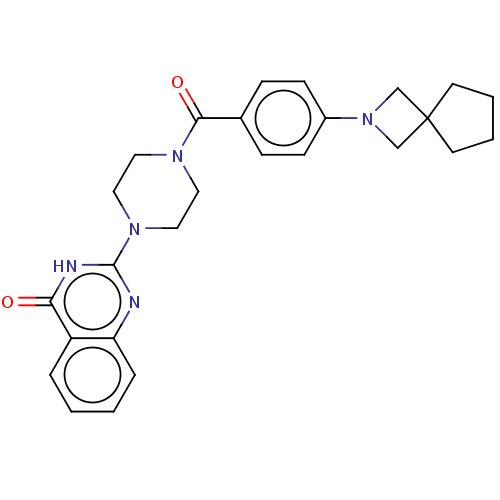
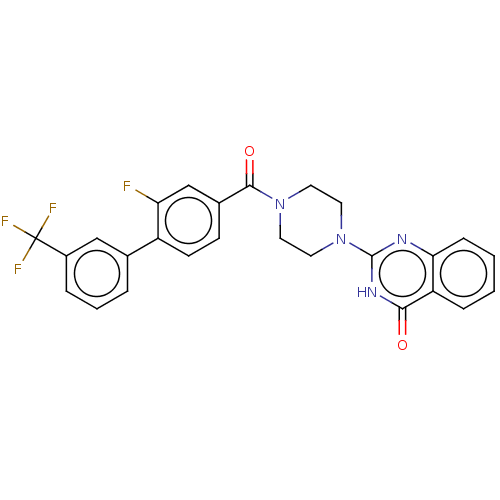
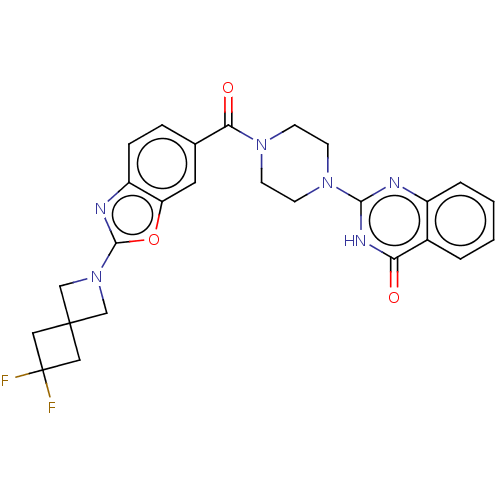
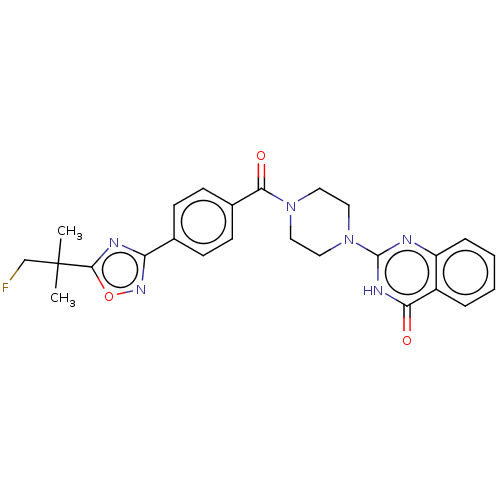
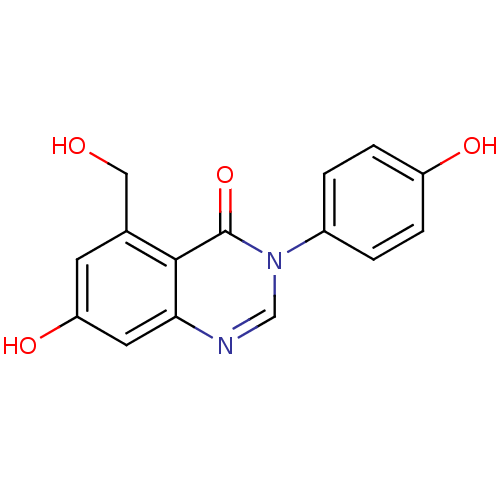
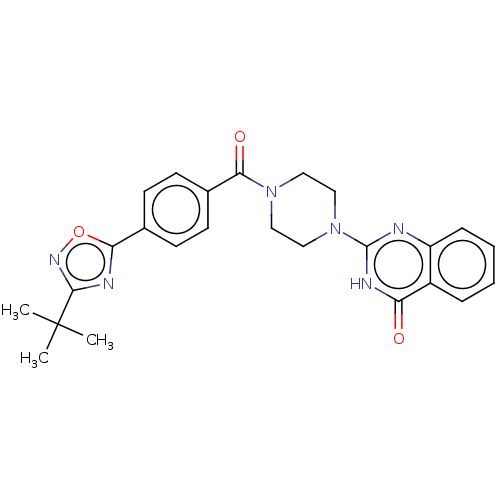
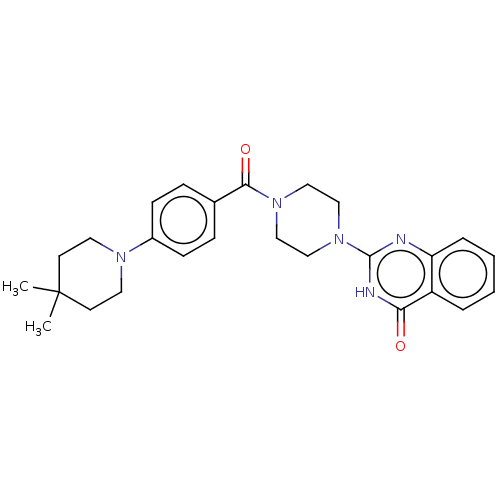
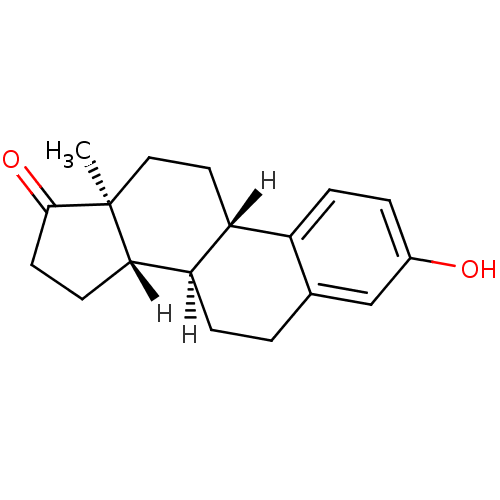
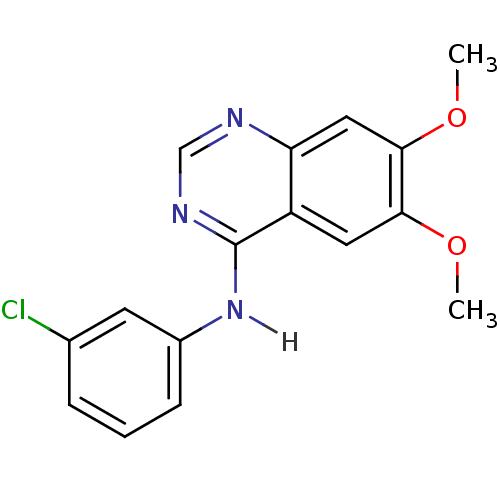
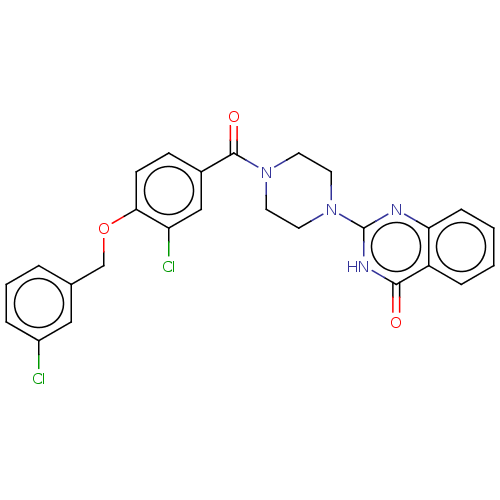
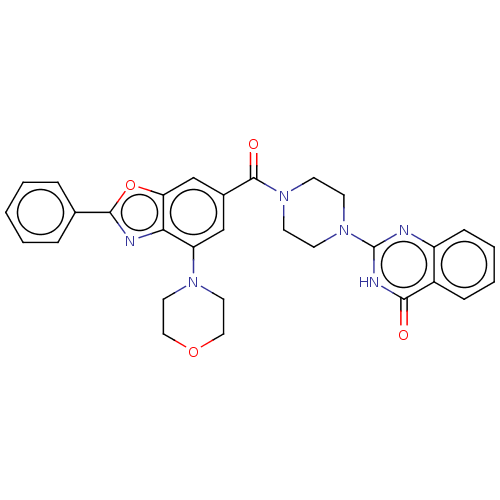
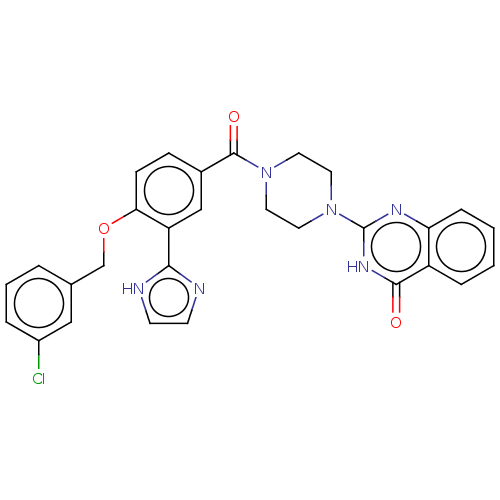
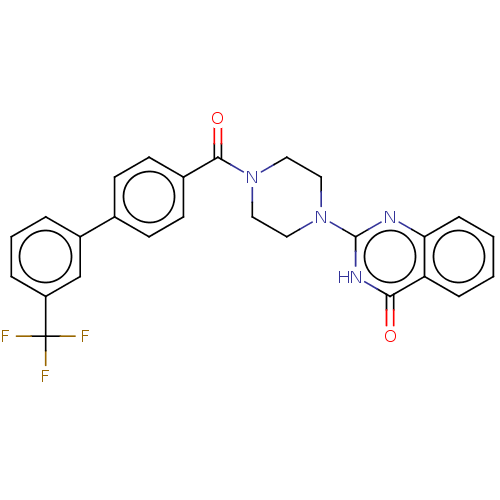
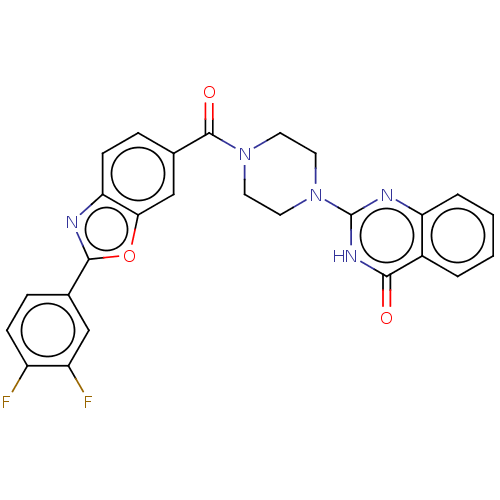
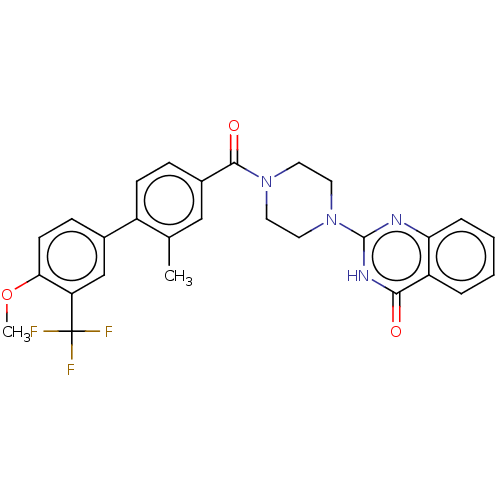
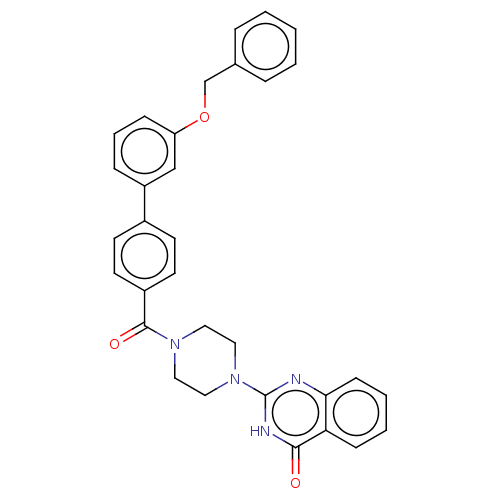
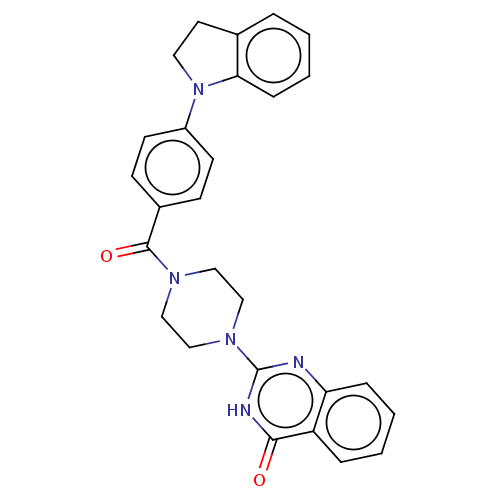
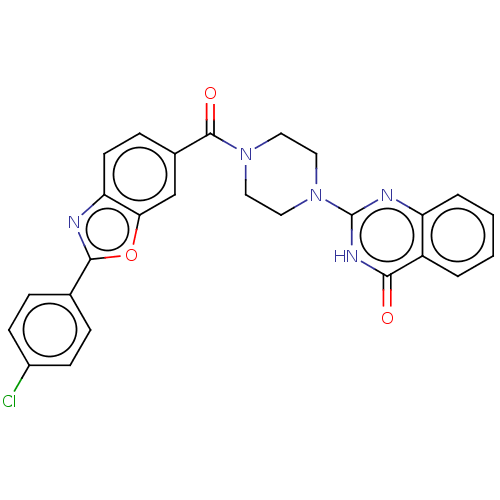
Affinity DataIC50: 3nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited.More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited.More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nM EC50: 0.590nMAssay Description:Ligand binding was determined using a scintillation proximity assay with streptavidin-coated SPA beads (Amersham) and biotinylated receptor. Receptor...More data for this Ligand-Target Pair
Affinity DataIC50: 11nM EC50: 0.840nMAssay Description:Ligand binding was determined using a scintillation proximity assay with streptavidin-coated SPA beads (Amersham) and biotinylated receptor. Receptor...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited.More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation.More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 24nM EC50: 4.46E+3nMAssay Description:Ligand binding was determined using a scintillation proximity assay with streptavidin-coated SPA beads (Amersham) and biotinylated receptor. Receptor...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation.More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 29nM EC50: 166nMAssay Description:Ligand binding was determined using a scintillation proximity assay with streptavidin-coated SPA beads (Amersham) and biotinylated receptor. Receptor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation.More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 31nM EC50: 26nMAssay Description:Ligand binding was determined using a scintillation proximity assay with streptavidin-coated SPA beads (Amersham) and biotinylated receptor. Receptor...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ...More data for this Ligand-Target Pair

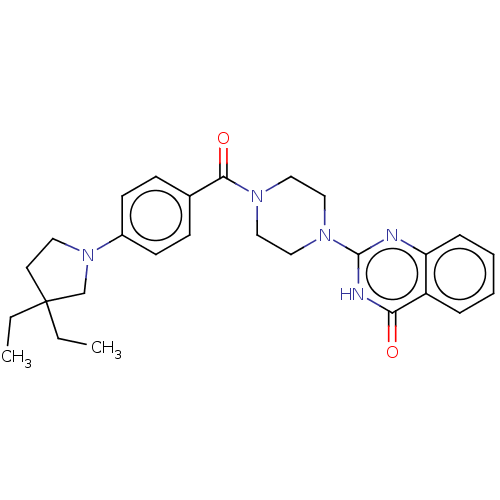
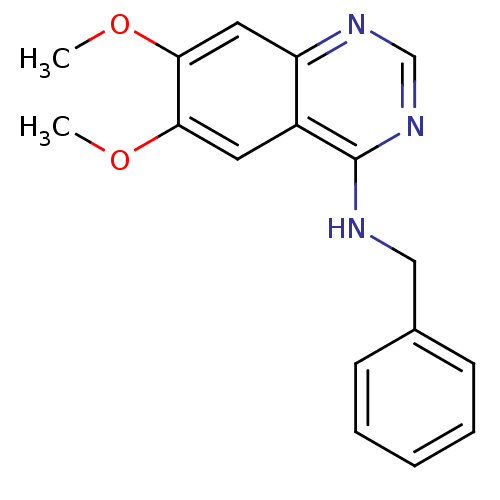
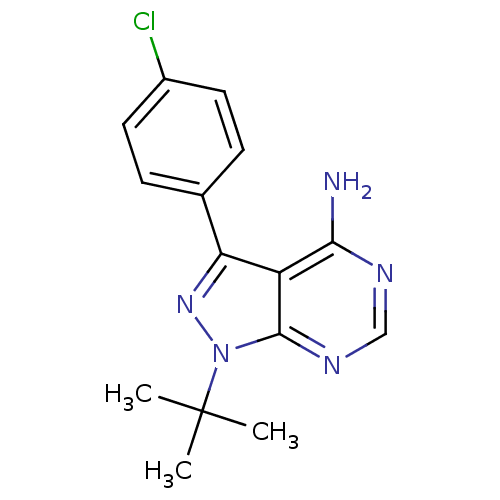
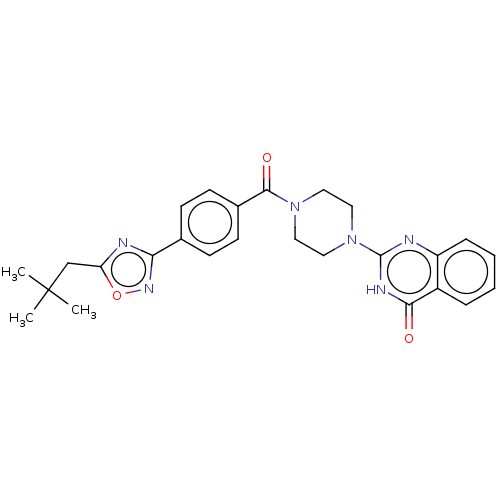

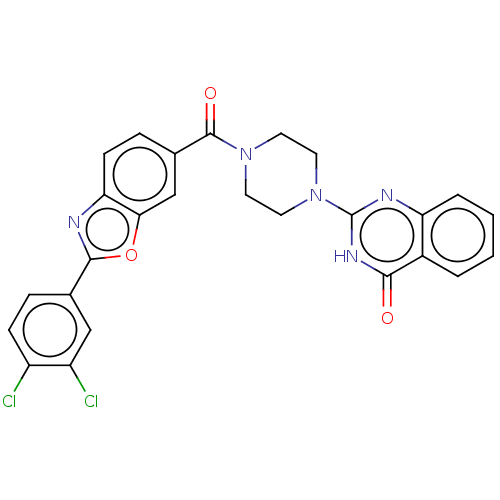
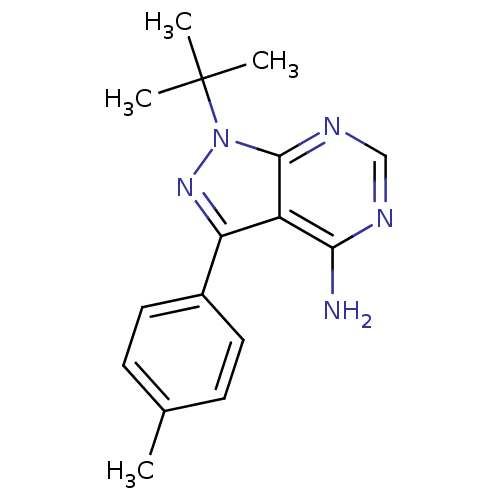
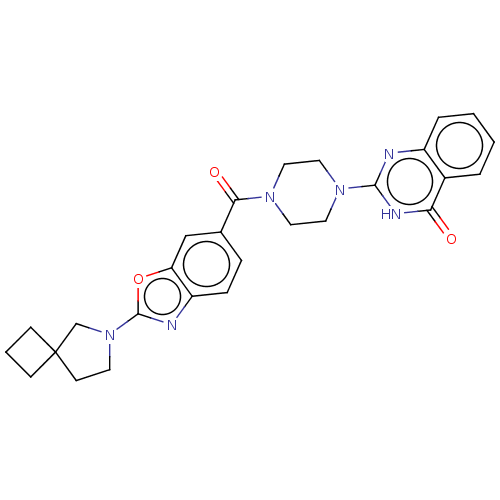
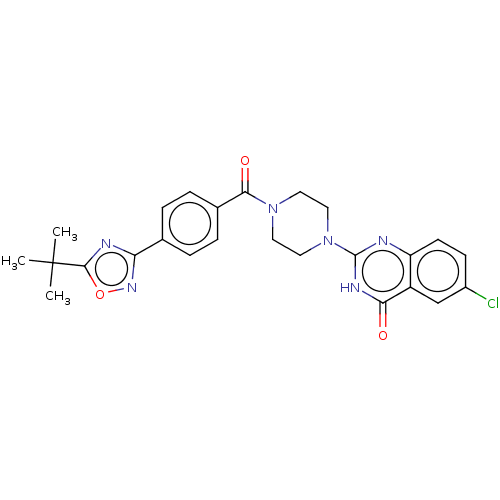
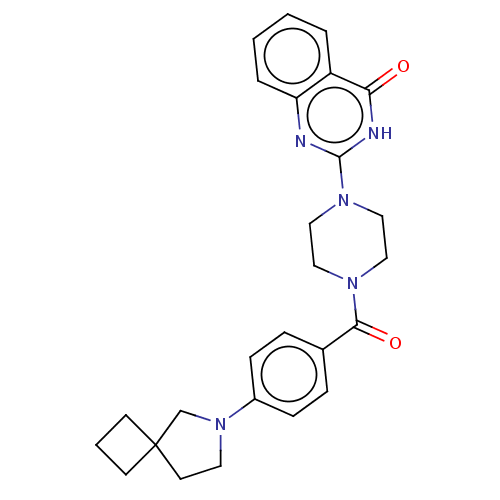

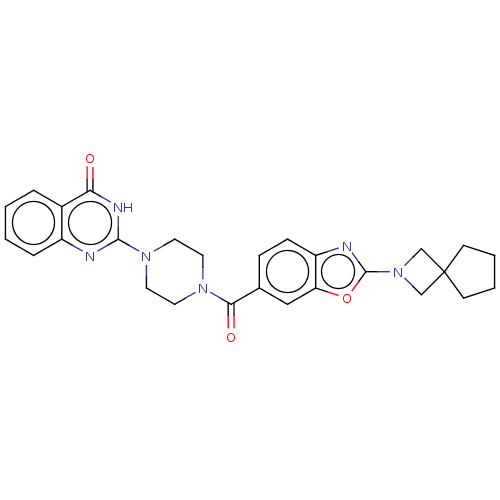
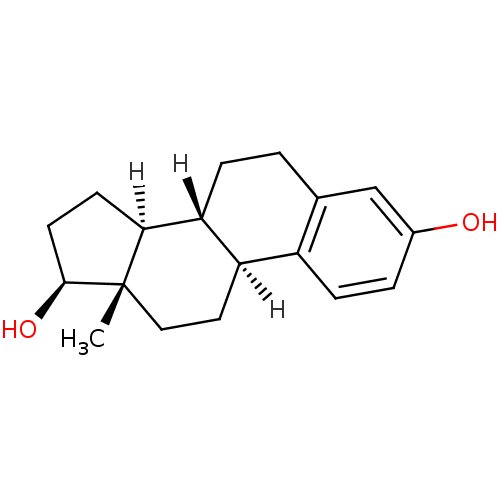
 3D Structure (crystal)
3D Structure (crystal)