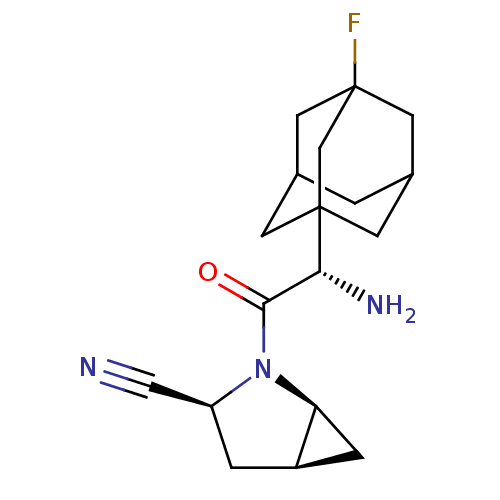
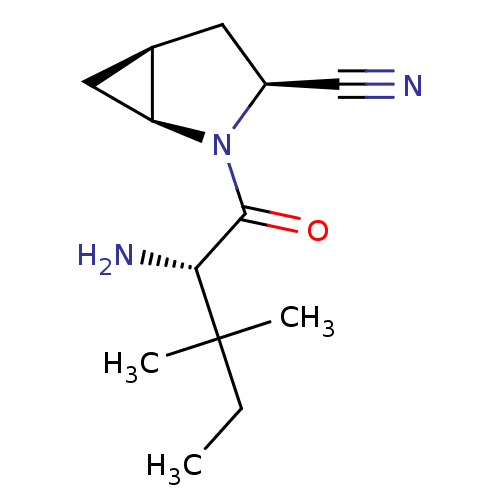
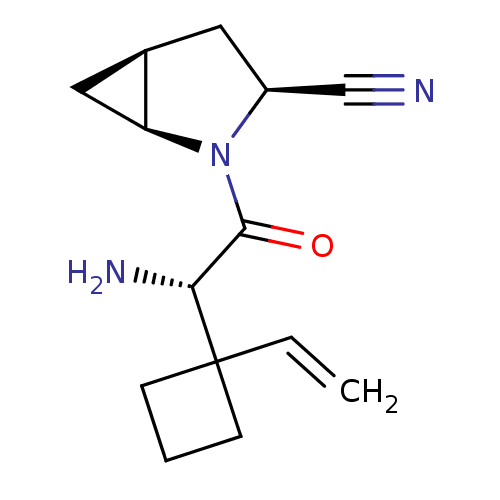
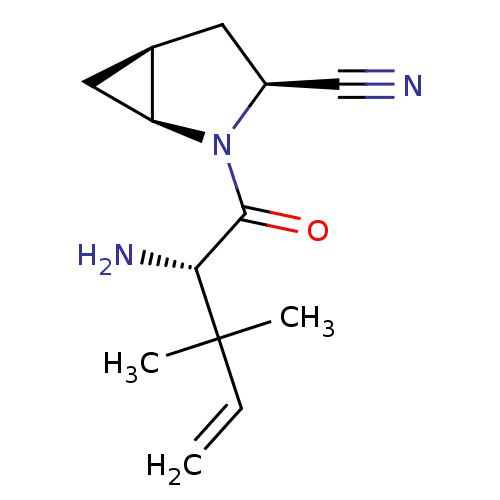
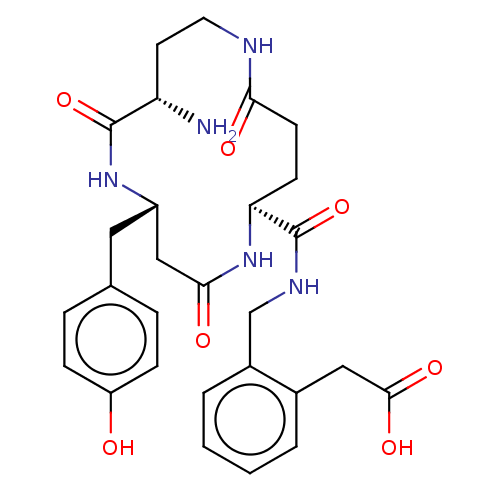
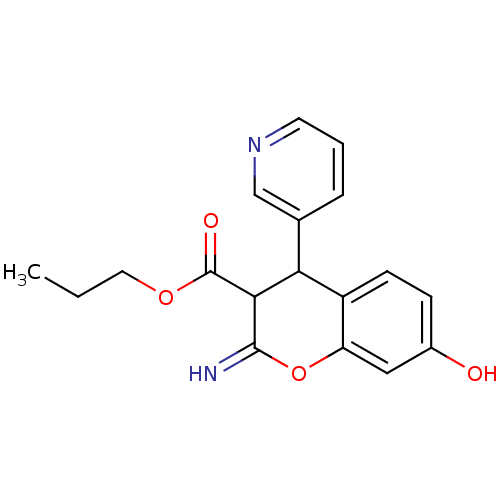
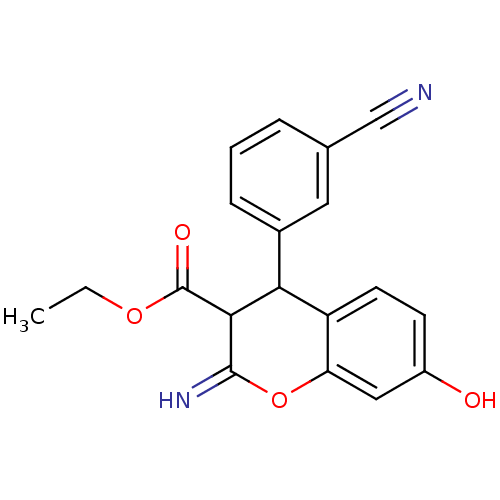
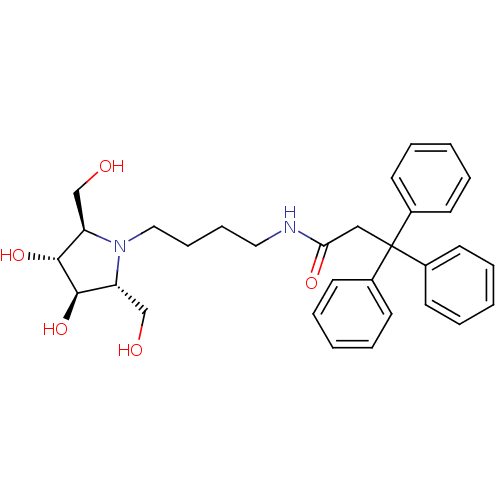
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
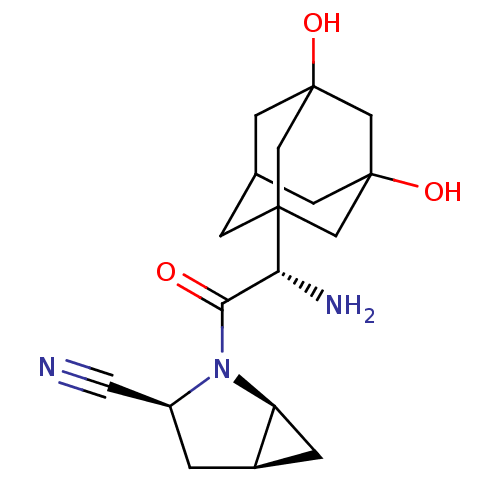
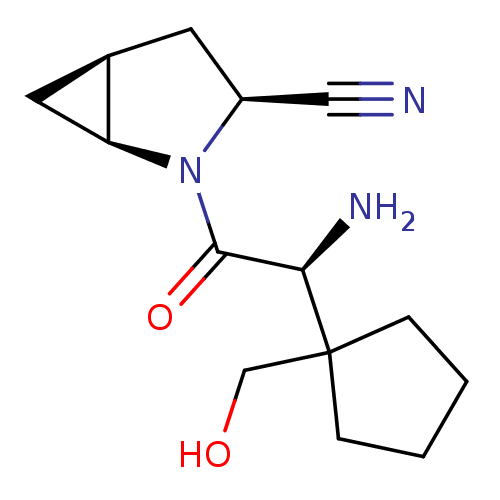
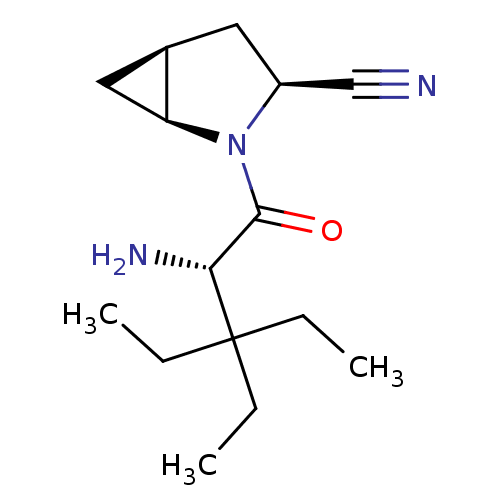
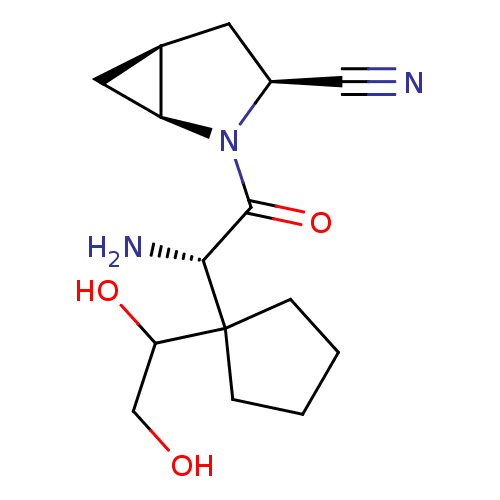
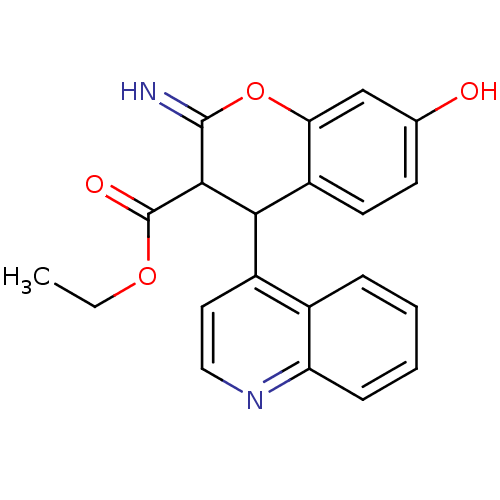
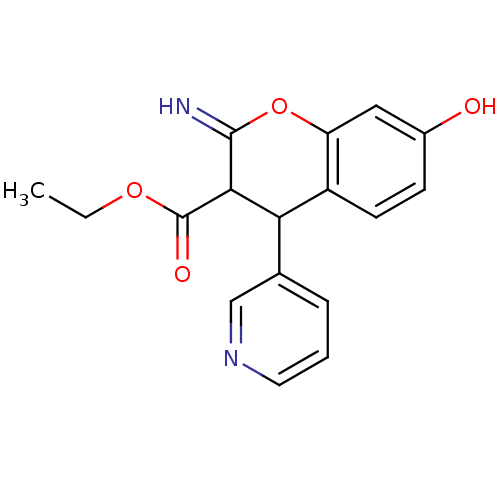
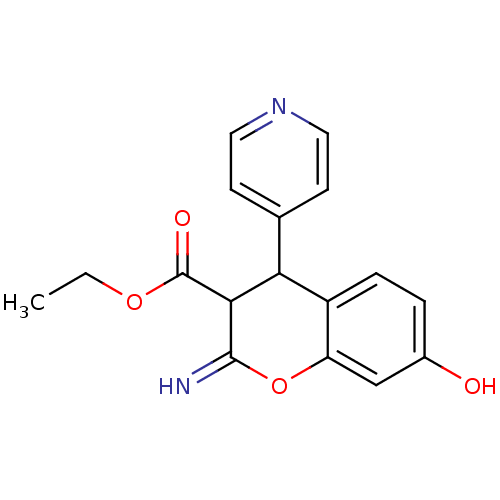
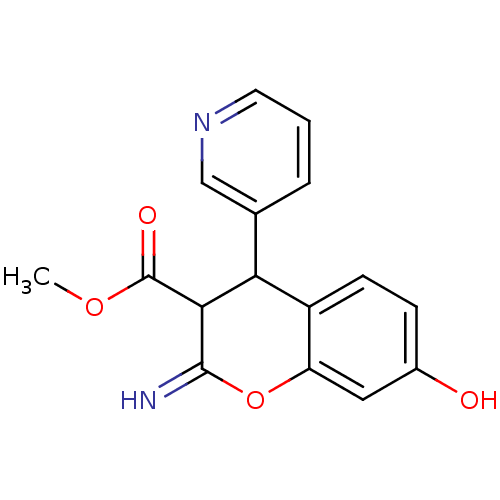
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 0.900nM ΔG°: -51.1kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
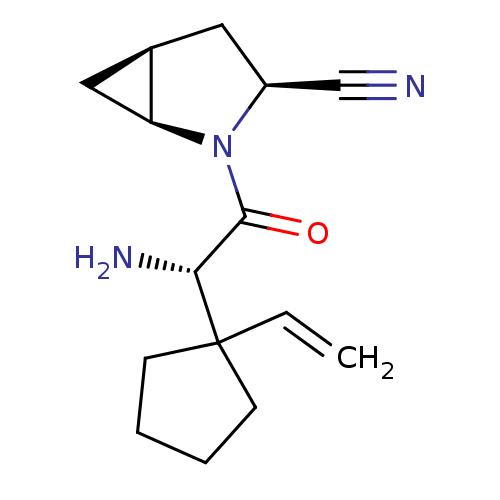
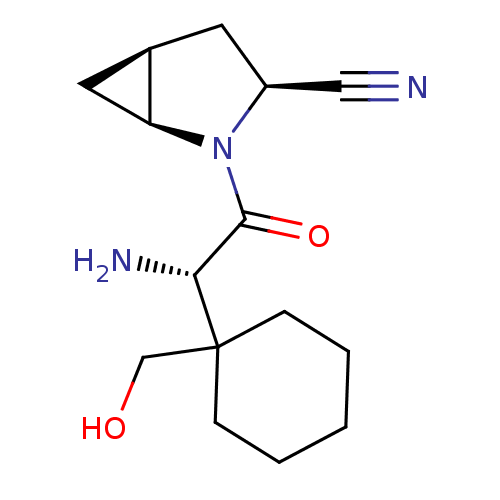
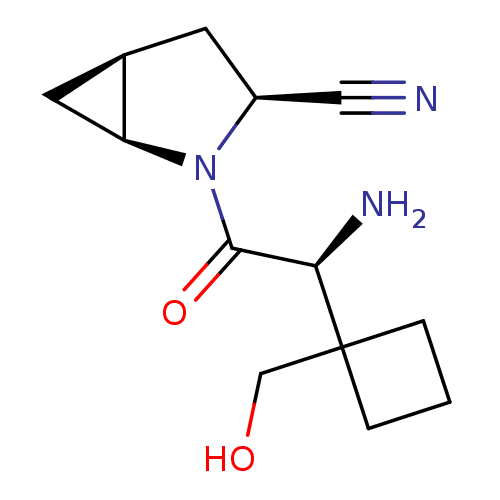
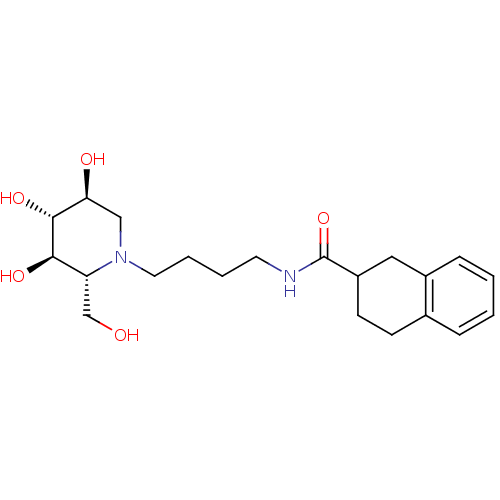
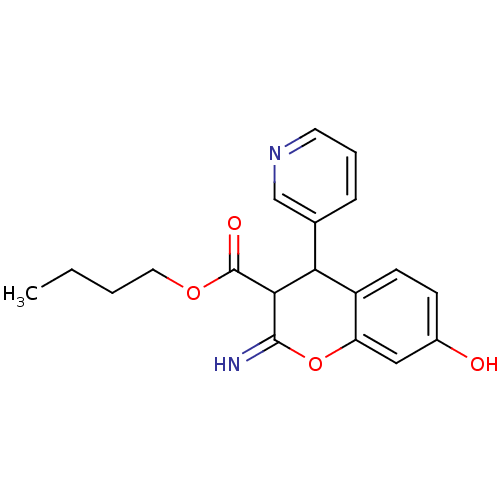
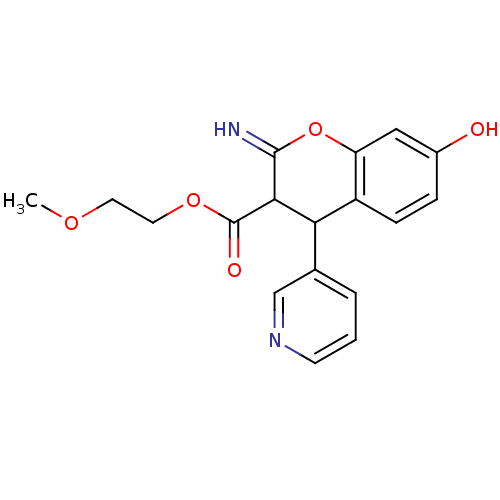
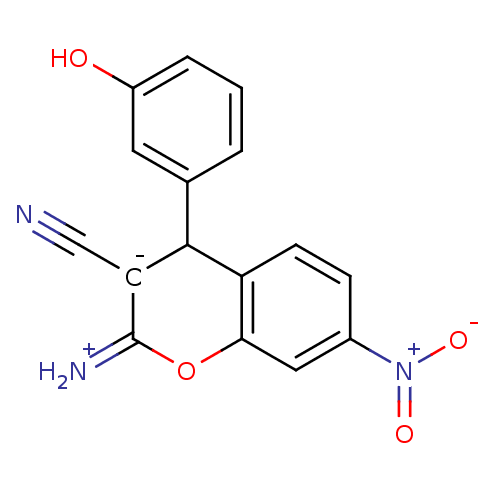
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 1.40nM ΔG°: -50.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
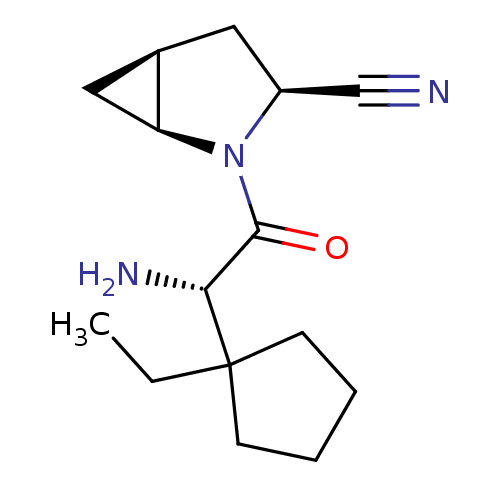
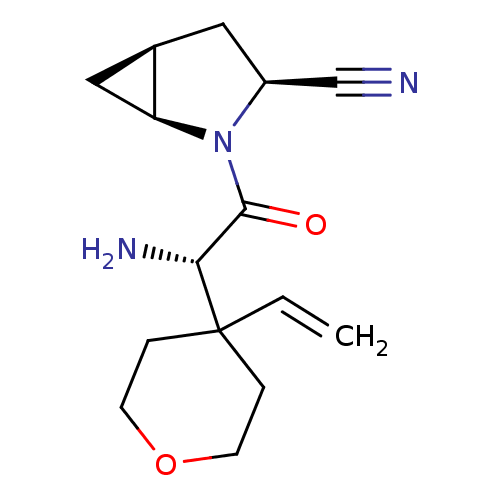
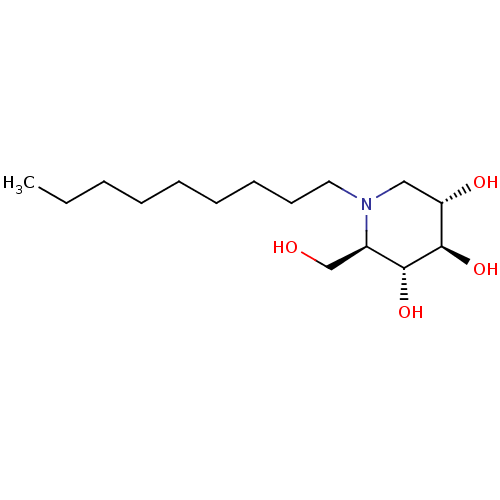
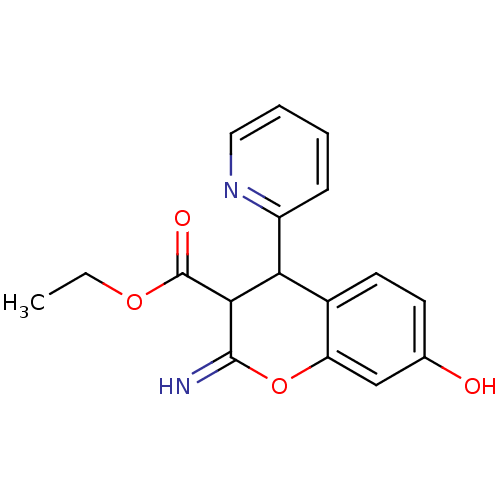
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 2.10nM ΔG°: -49.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 3.90nM ΔG°: -47.5kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 5.5nM ΔG°: -46.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 7.10nM ΔG°: -46.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 7.40nM ΔG°: -45.9kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 8nM ΔG°: -45.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 10nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 10nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
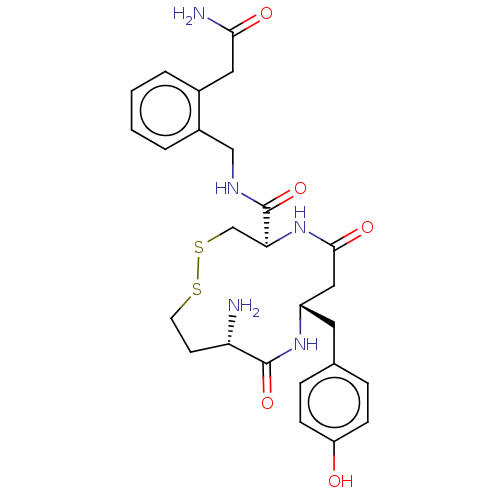
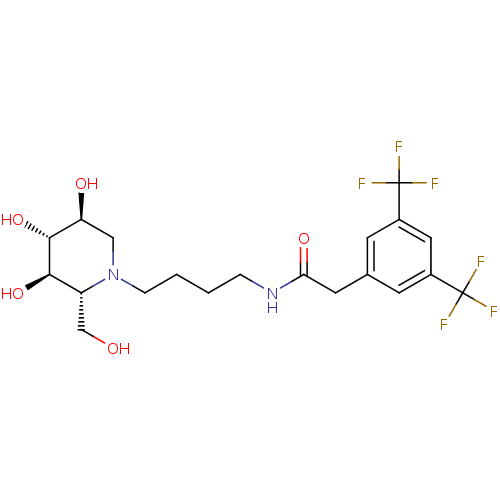
Affinity DataKi: 11nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 12nM ΔG°: -44.8kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 21nM ΔG°: -43.4kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 25nM ΔG°: -43.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
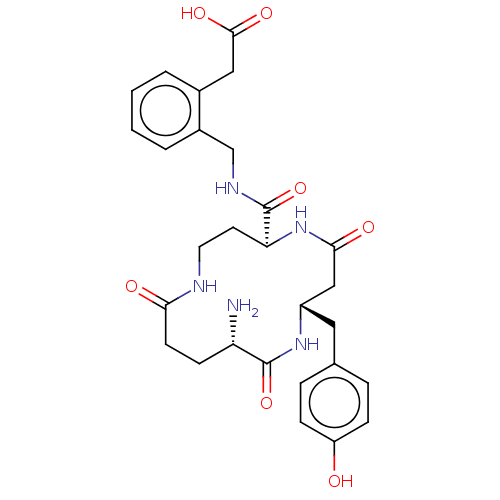
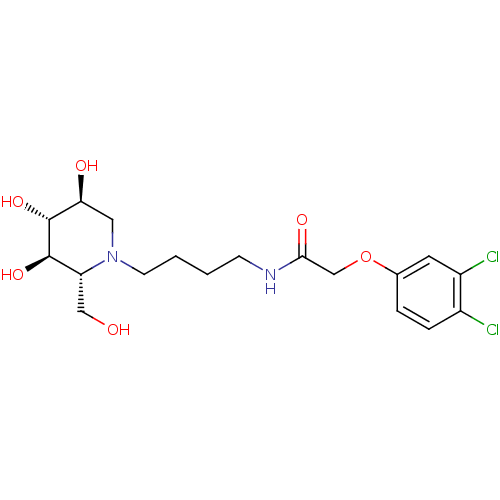
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 31nM ΔG°: -42.4kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 42nM ΔG°: -41.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 57nM ΔG°: -40.9kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 71nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 143nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 201nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 480nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 674nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 887nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.25E+3nMAssay Description:Binding affinity to recombinant full length human IRAP expressed in HEK293T cells using Leu-MCA as substrateMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 4.10E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 4.90E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Monash Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 minsMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)