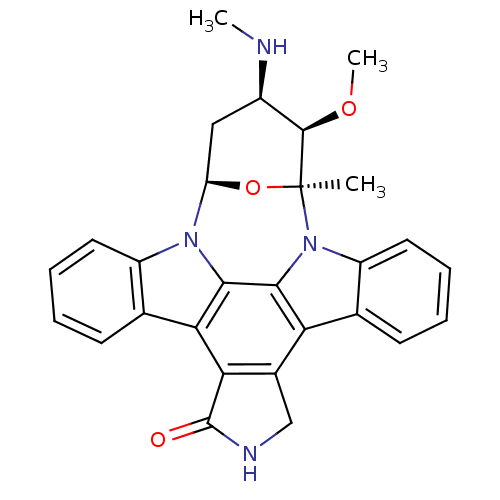
Affinity DataIC50: 0.751nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.61nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.77nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human PKCalpha activity using kemptide as a substrate in presence of 50 uM ATP by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.02nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.97nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.49nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.65nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.83nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.33nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 10.5nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 12.7nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 15.9nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 20.3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit beta(Homo sapiens (Human))
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of PKA activity using neurogranin as a substrate in presence of 50 uM ATP by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 39.6nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 43.3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 51.9nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 55.7nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 95nMAssay Description:Inhibition of human PKCalpha activity using kemptide as a substrate in presence of 50 uM ATP by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 153nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 166nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 354nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 366nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 376nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 567nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 840nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 927nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 970nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.33E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.67E+3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.71E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.82E+3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.84E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.02E+3nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.15E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of human PKCalpha activity using kemptide as a substrate in presence of 50 uM ATP by mass spectrometryMore data for this Ligand-Target Pair

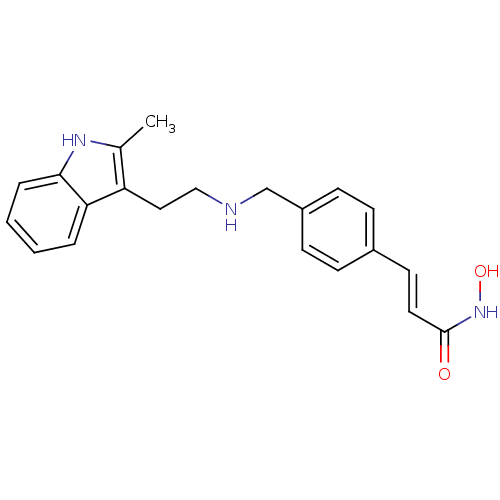
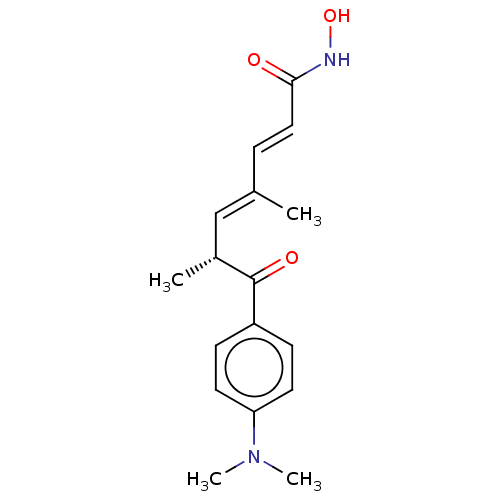
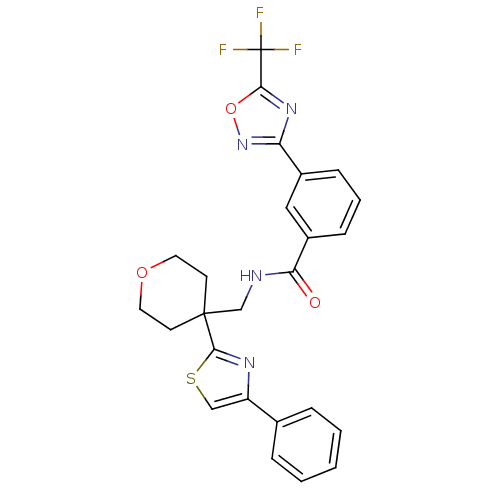
 3D Structure (crystal)
3D Structure (crystal)