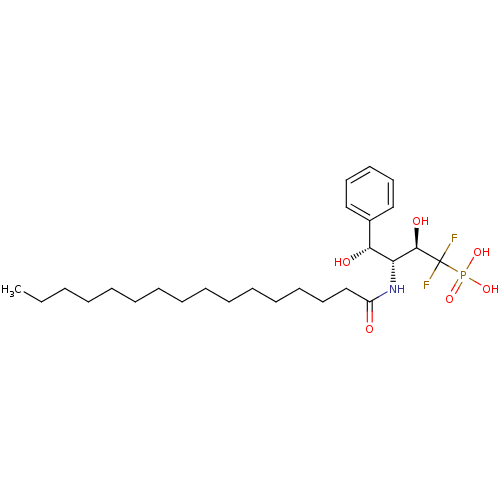
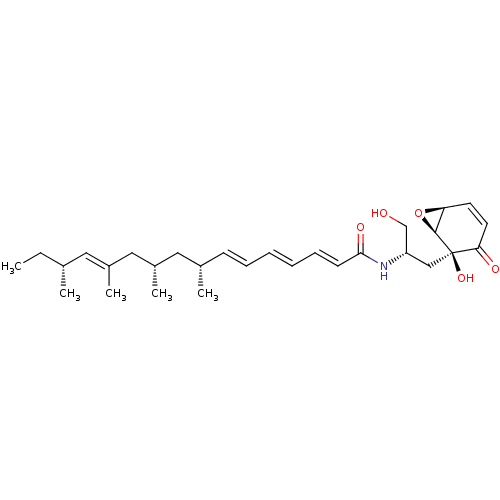
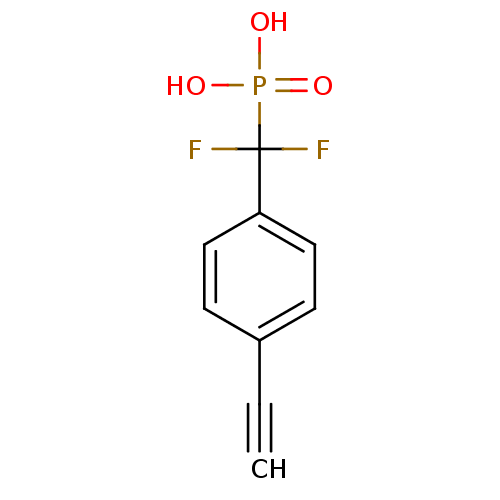
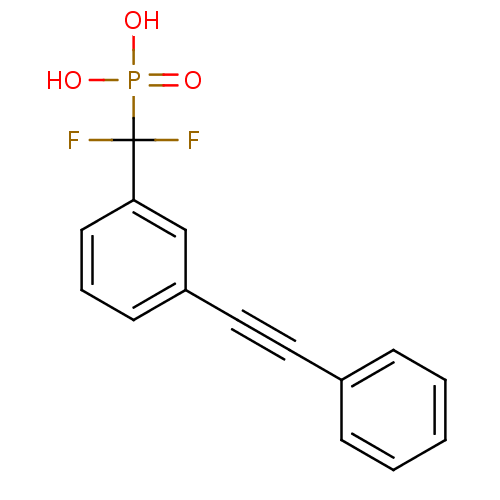
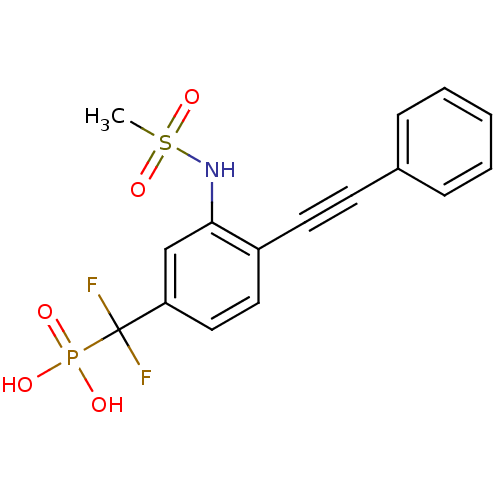
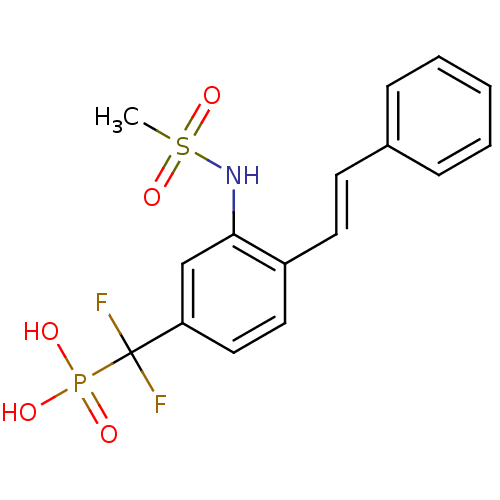
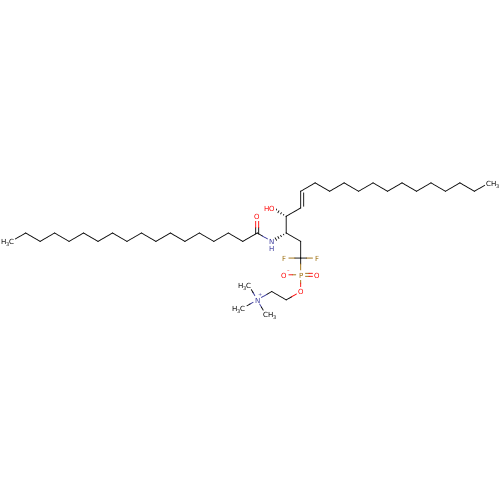
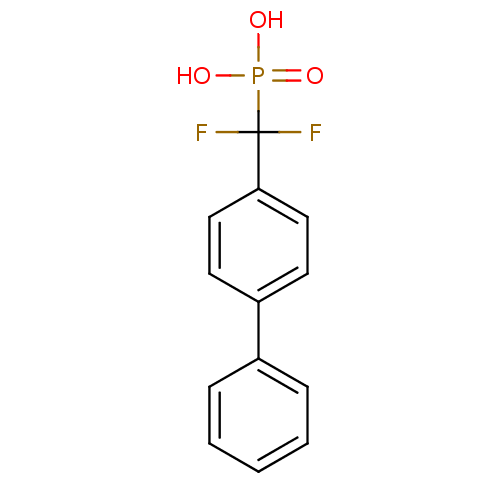
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibitory activity of the compound against schyphostatin of neutral sphingomyelinase (N-SMase) from bovine brain microsomeMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 2.53E+5nMAssay Description:Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase [1-45,48-631](Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 4.90E+4nMAssay Description:Inhibitory activity of the compound against Acid sphingomyelinase from bovine brain microsomeMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 5.79E+4nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 7.75E+4nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 8.87E+4nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.28E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.36E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.67E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.76E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.81E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 1.81E+5nMAssay Description:Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 3.77E+5nMAssay Description:Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 3.77E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 3.86E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 4.00E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 4.50E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 4.51E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 7.18E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 7.79E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair