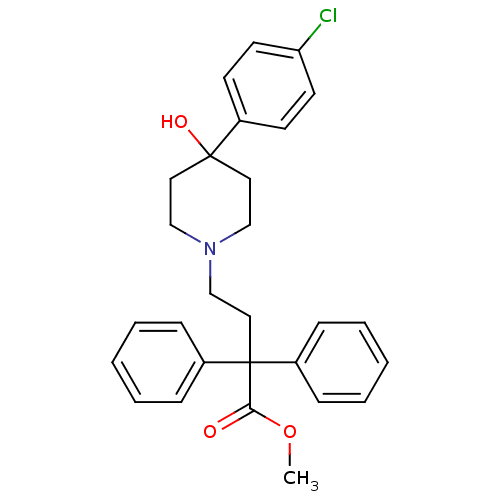
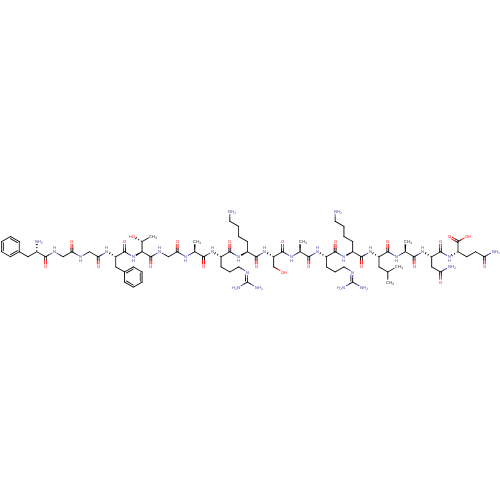
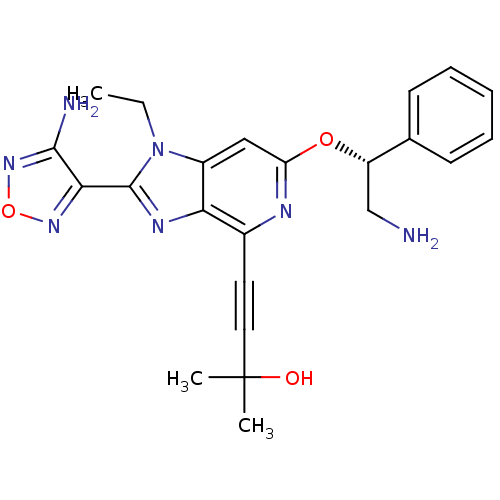
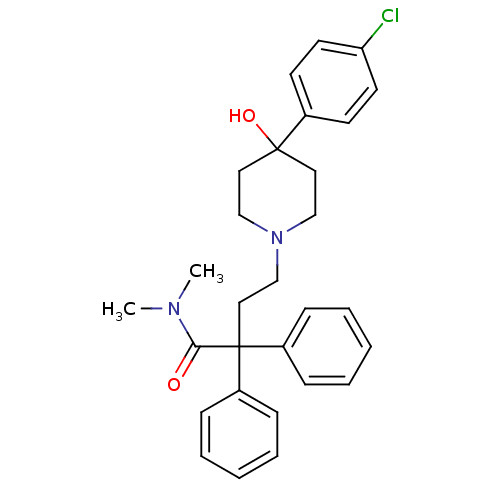
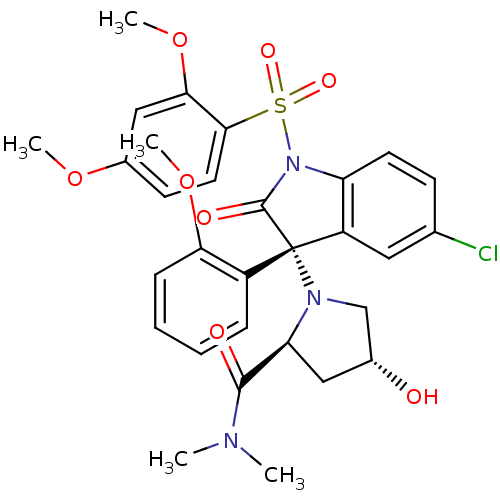
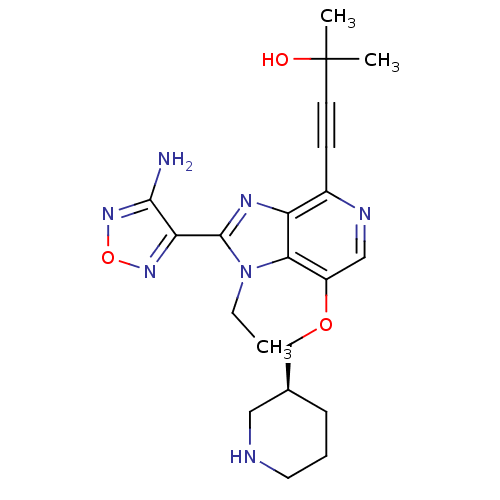
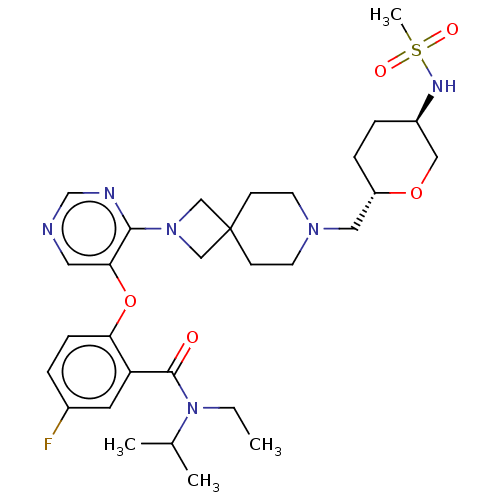
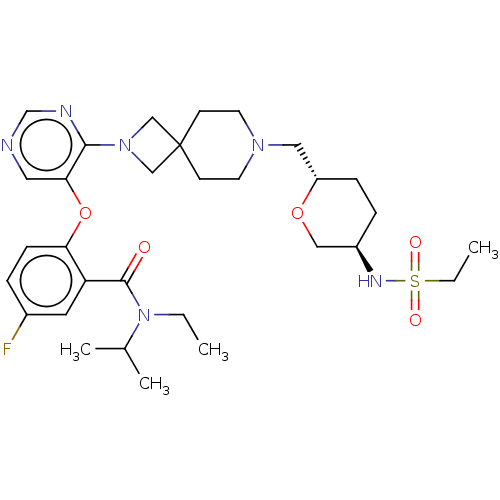
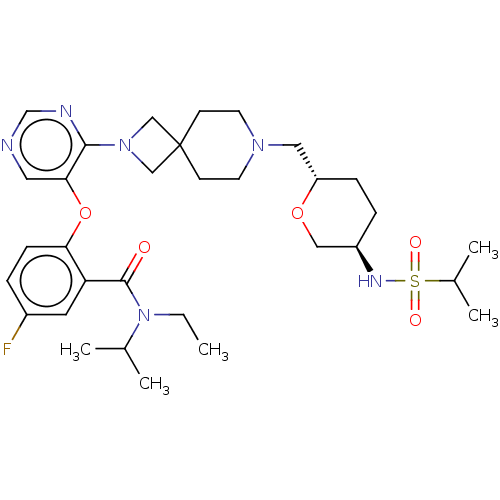
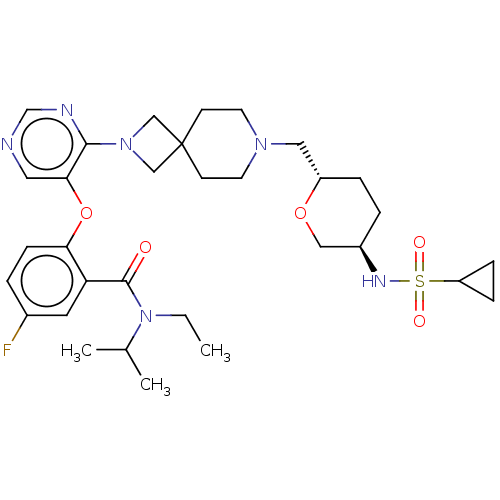
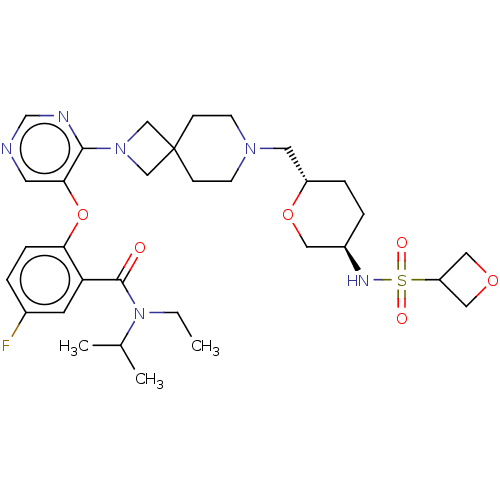
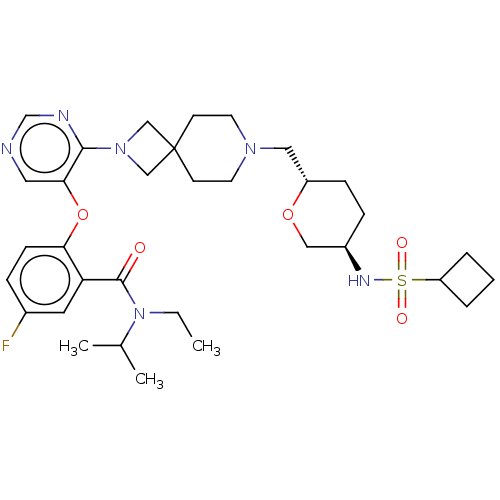
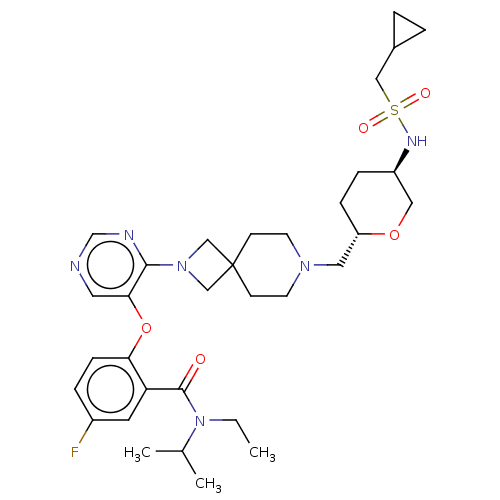
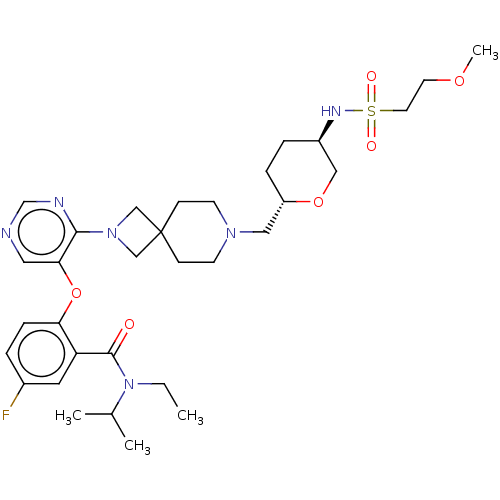
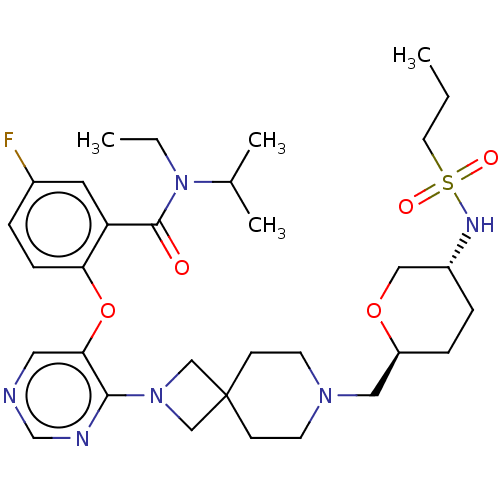
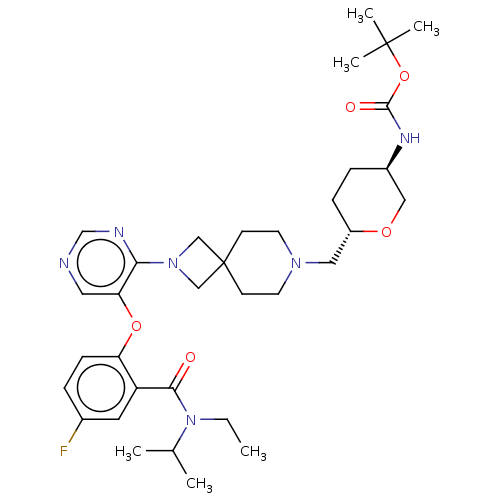
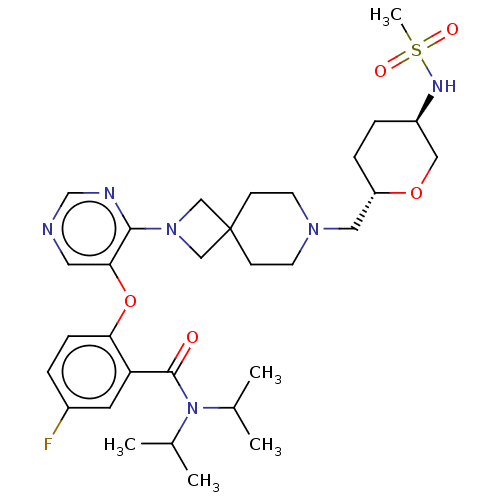
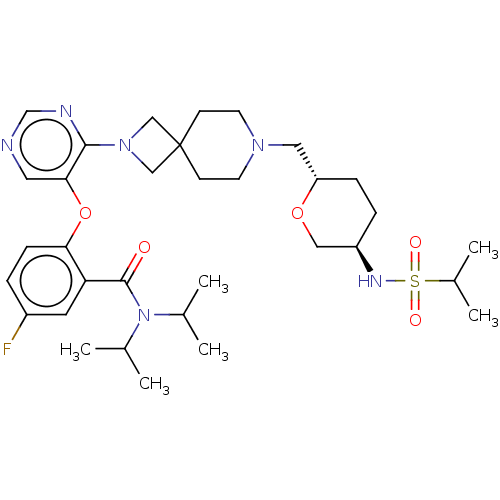
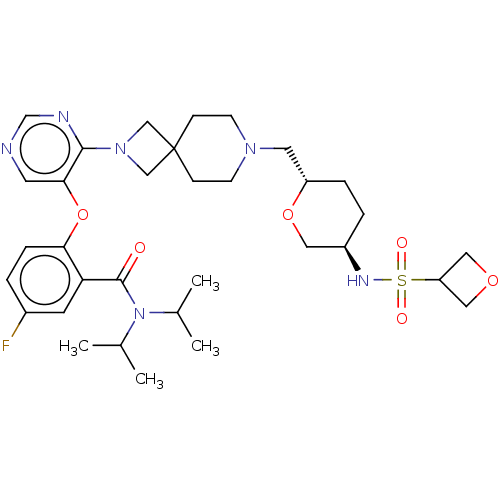
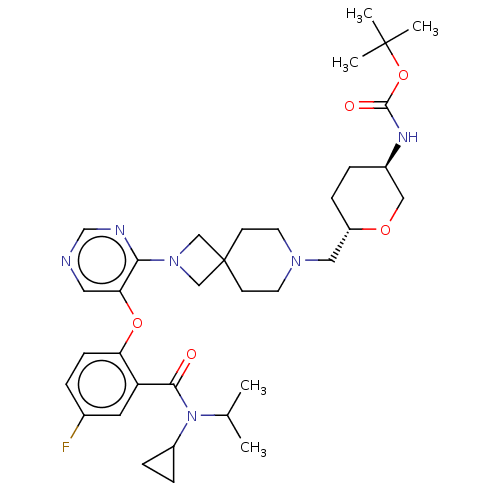
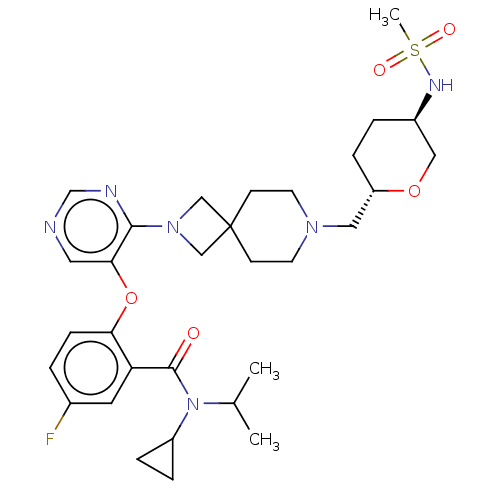
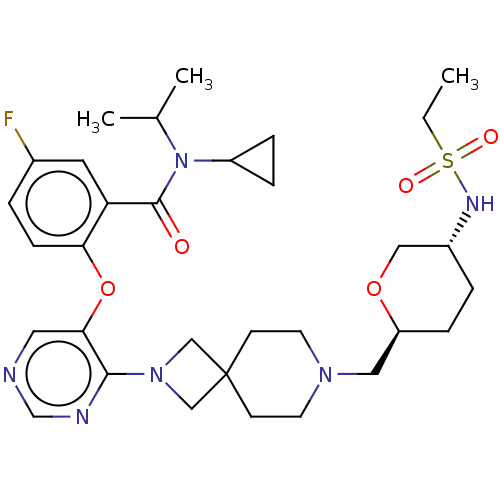
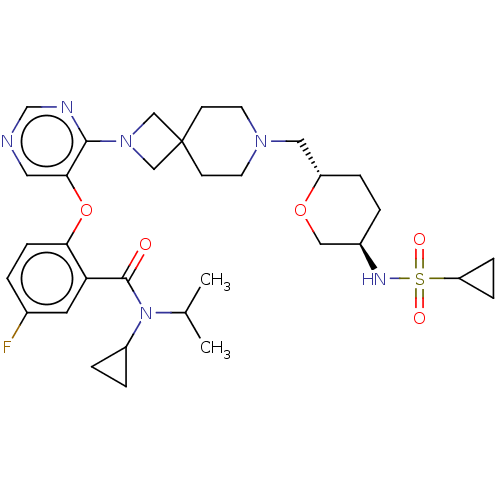
Affinity DataKi: 0.0200nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Inhibition of [3H]nociceptin binding to human Opioid receptor like 1More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.480nMAssay Description:Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
TargetVasopressin V1b receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Binding affinity for Vitronectin receptor (alpha V beta 3)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:In vitro binding affinity against human alpha IIb beta3 integrinMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Antagonistic activity against opioid receptor mu1More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
TargetHistone-lysine N-methyltransferase 2A/Menin(Homo sapiens (Human))
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Syndax Pharmaceuticals, Inc.; Vitae Pharmaceuticals, LLC
US Patent
Affinity DataKi: <1nMAssay Description:Table 4: For Ki determination, individual compounds were prepared as 10 mM DMSO stock solutions. Considering DMSO as the vehicle in the assay system....More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1nMAssay Description:Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair



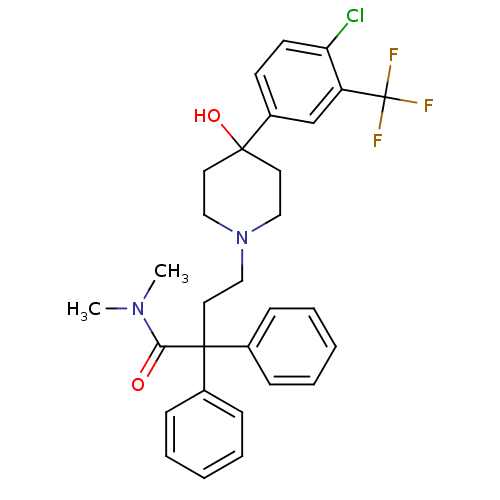



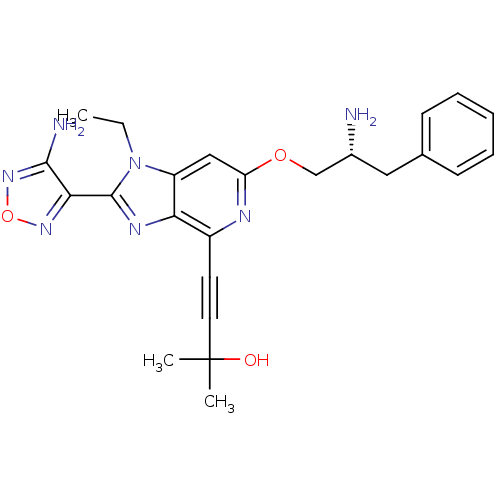
 3D Structure (crystal)
3D Structure (crystal)