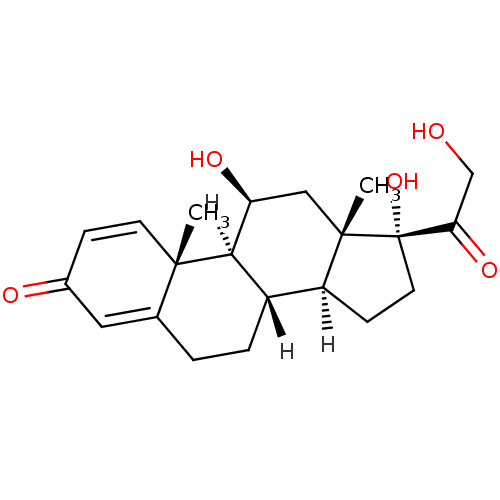
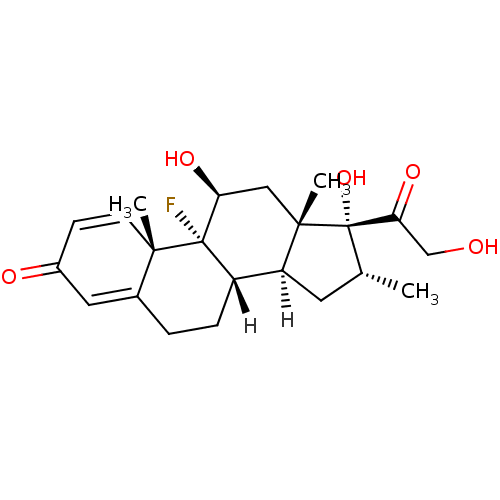
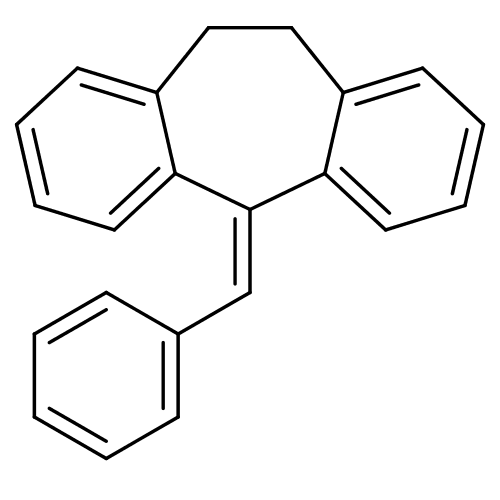
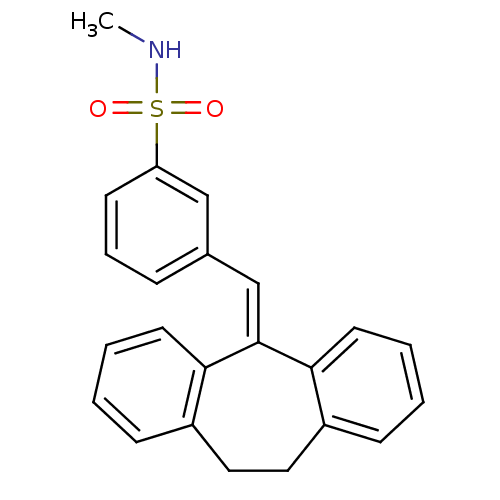
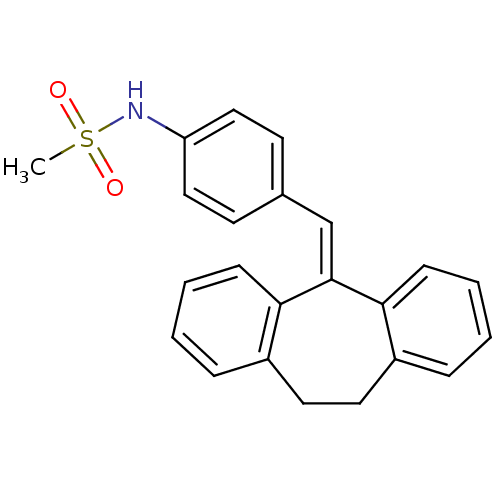
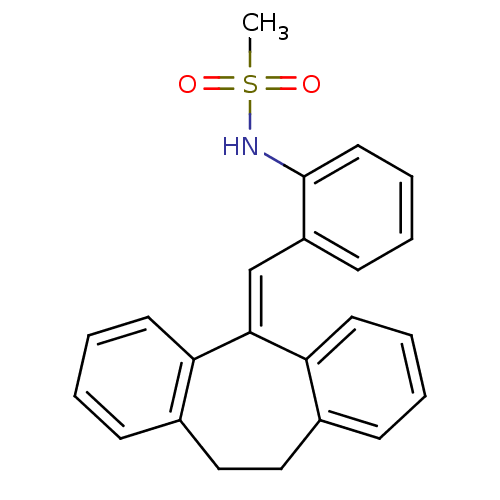
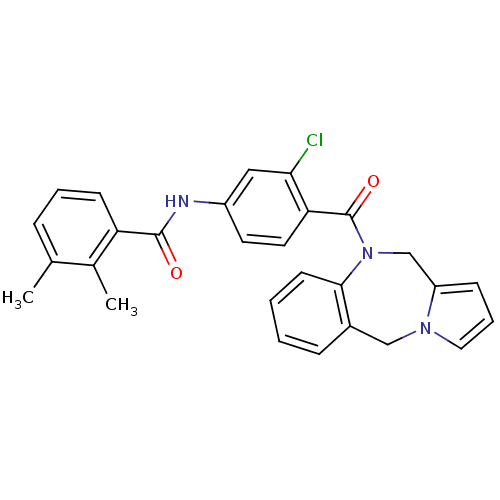
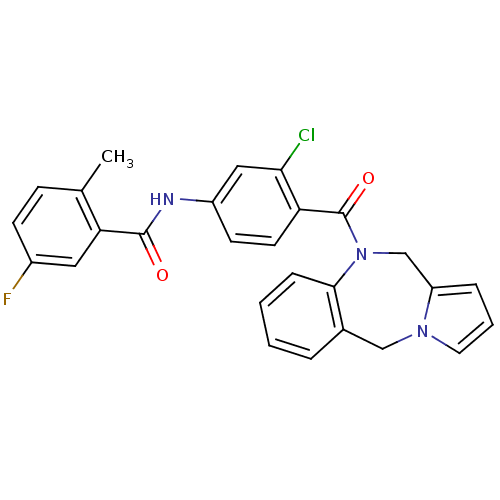
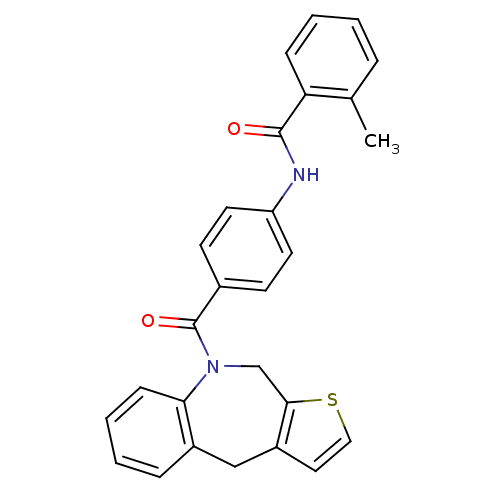
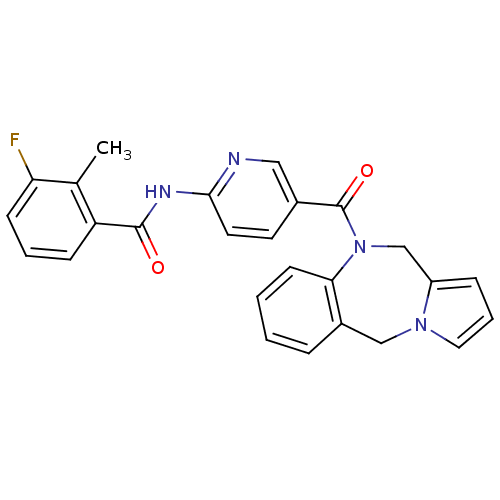
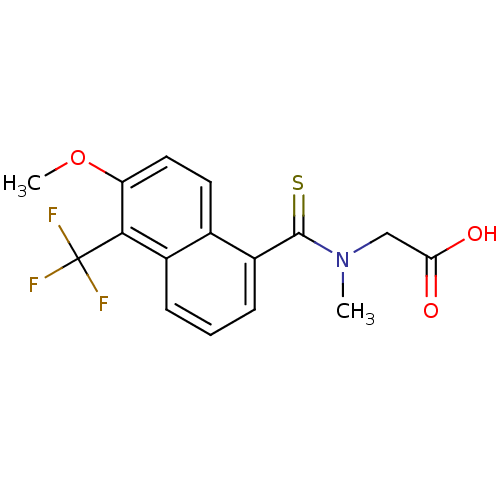
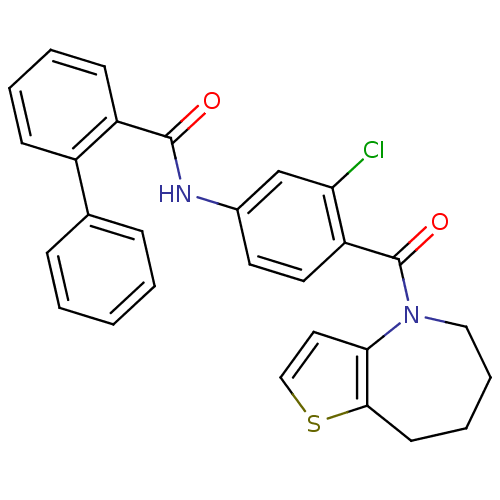
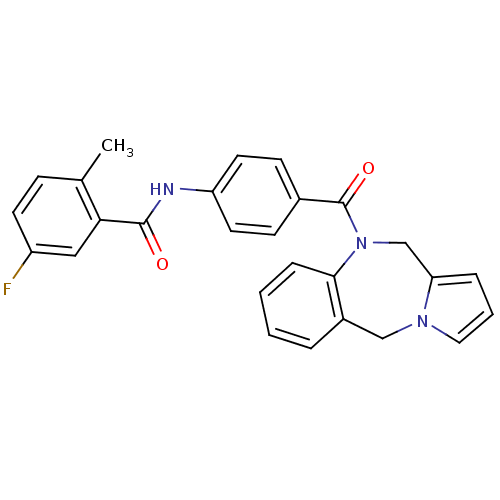
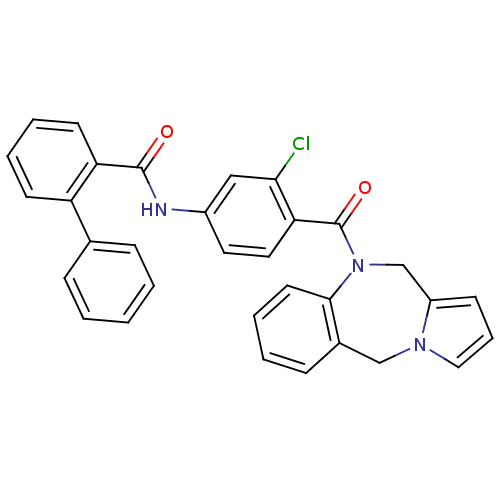
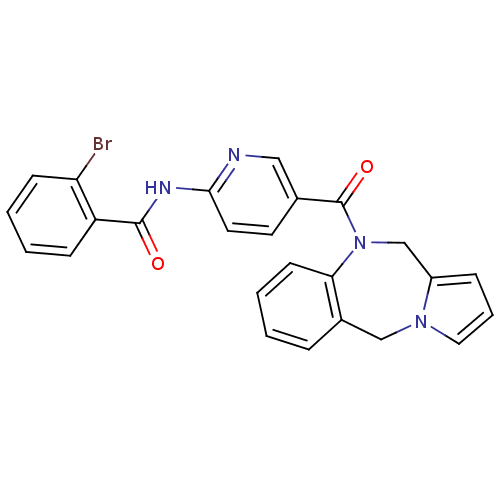
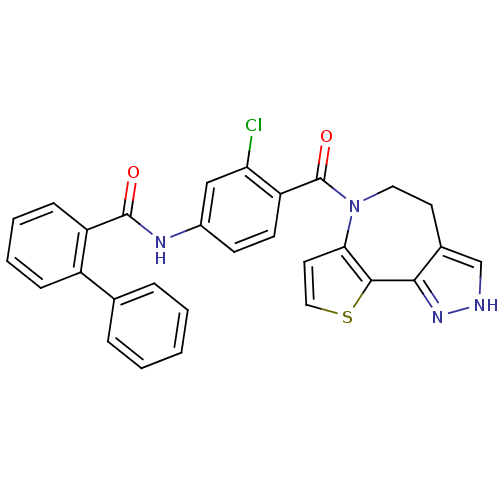
Affinity DataKi: 0.146nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.226nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.257nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.268nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.290nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.342nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.360nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.967nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.70nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 99nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 239nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 418nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 561nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.41E+3nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.52E+3nMAssay Description:Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of [3H]-AVP binding to human V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptorMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Institut De Ge??Ne??Tique Et De Biologie Mole??Culaire Et Cellulaire
Institut De Ge??Ne??Tique Et De Biologie Mole??Culaire Et Cellulaire
Affinity DataIC50: 1nMpH: 7.0Assay Description:The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptorMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Institut De Ge??Ne??Tique Et De Biologie Mole??Culaire Et Cellulaire
Institut De Ge??Ne??Tique Et De Biologie Mole??Culaire Et Cellulaire
Affinity DataIC50: 1.20nMpH: 7.0Assay Description:The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vitro binding affinity to human V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Binding affinity to rat V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity to rat V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In vitro binding affinity to human V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptorMore data for this Ligand-Target Pair

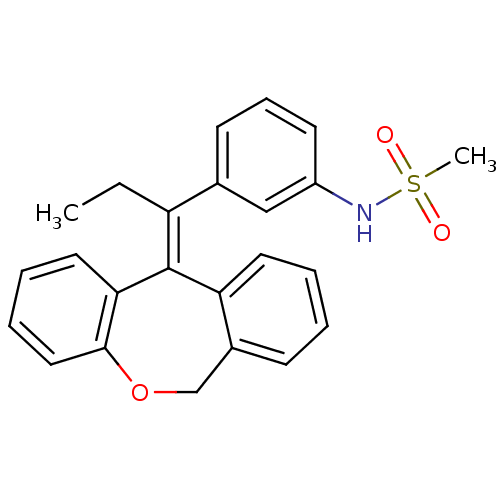
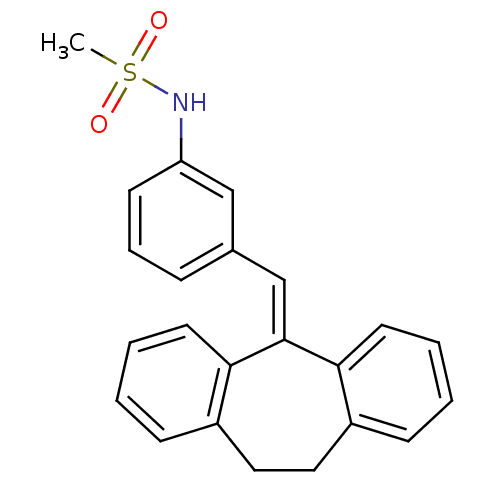
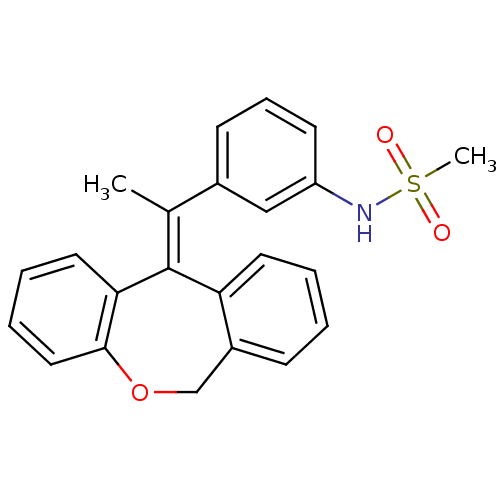
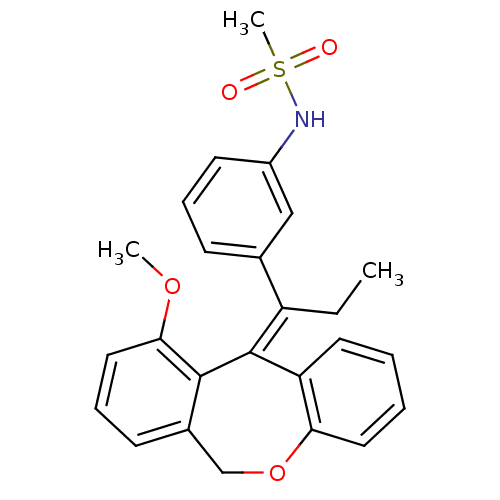
 3D Structure (crystal)
3D Structure (crystal)