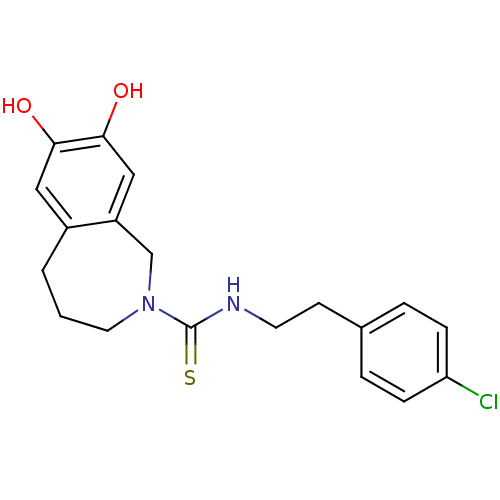
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(GUINEA PIG)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to guinea pig histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Displacement of [3H]resiniferatoxin from human Transient receptor potential vanilloid 1 receptor; (n=2)More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Binding affinity to rat histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 71nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of acid activated rat TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of acid activated rat TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of acid activated rat TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of acid activated rat TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of anandamide activated human TRPV1 receptor in [Ca2+] influx assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of human transient receptor potential vanilloid 1 receptor (n=6)More data for this Ligand-Target Pair

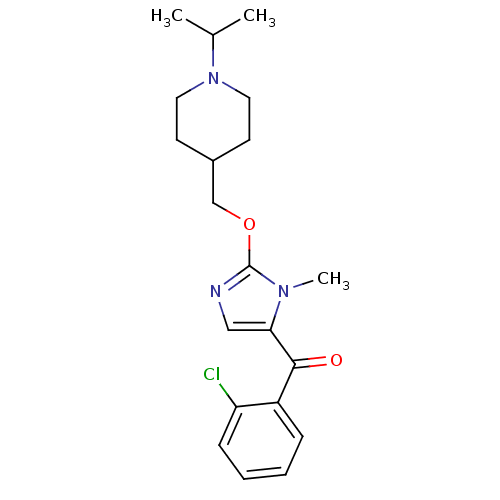
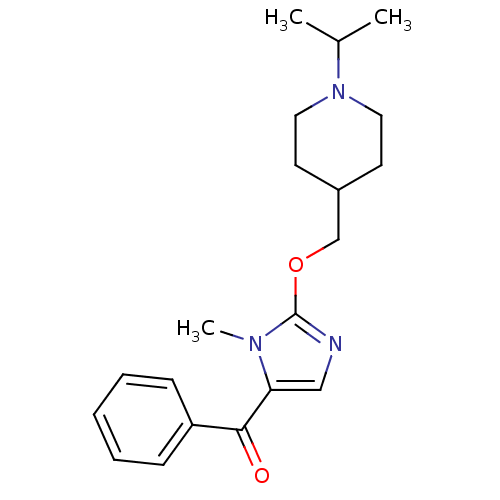
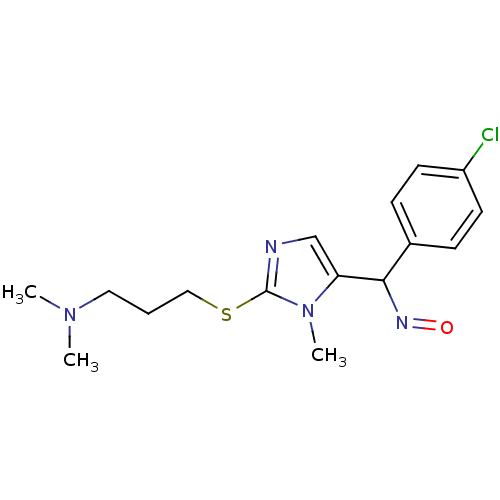
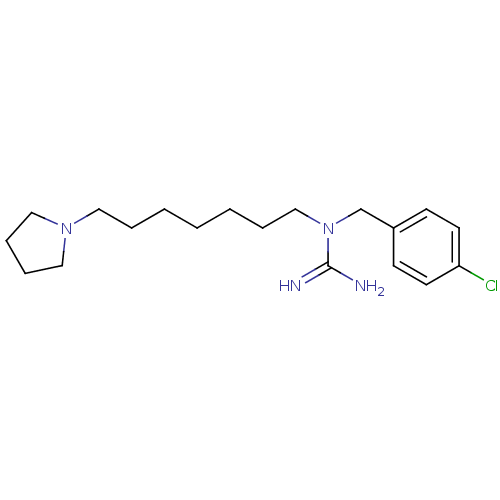
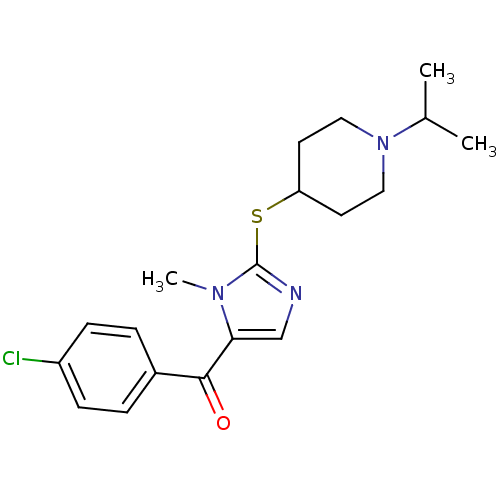
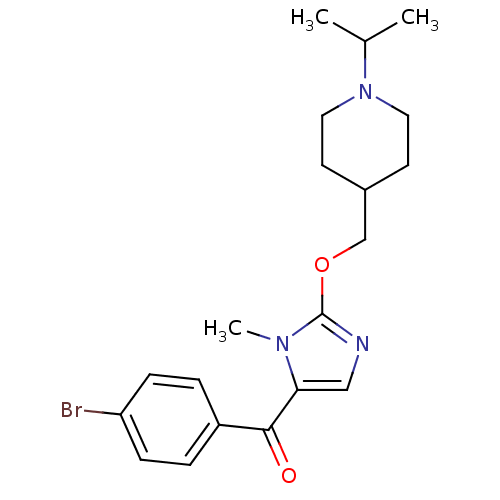
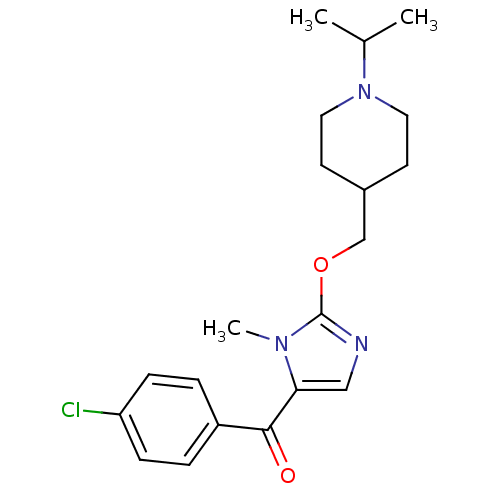
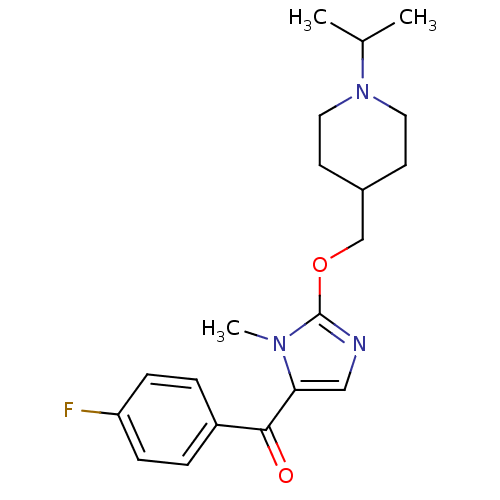
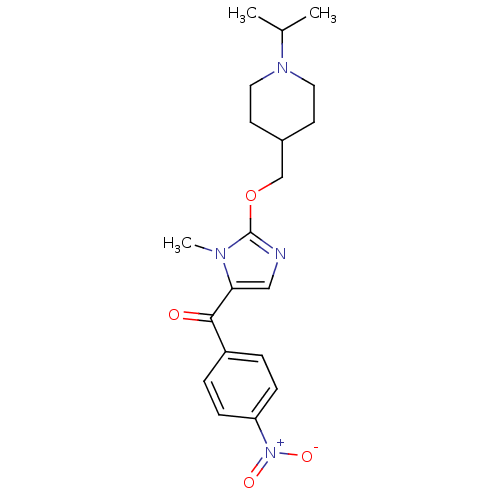
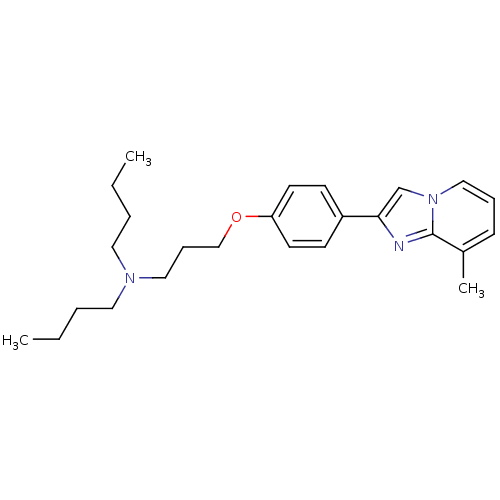
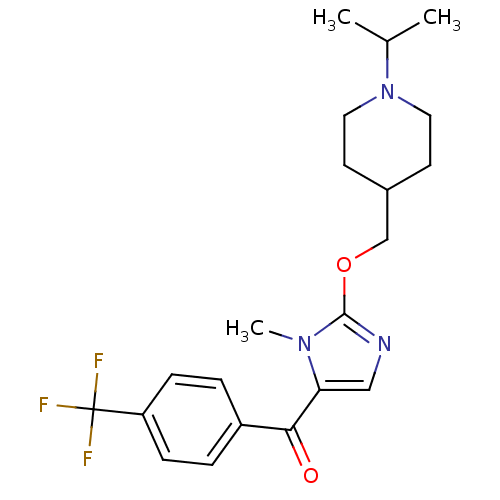
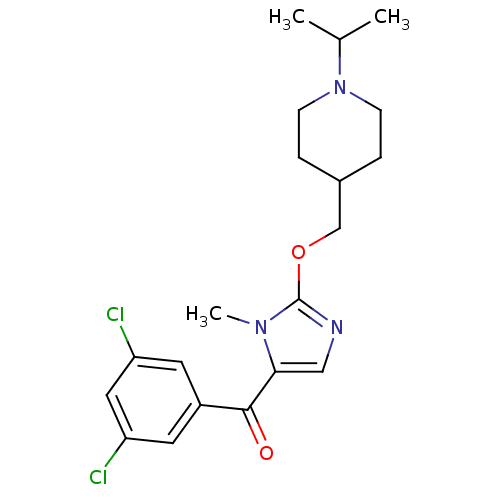
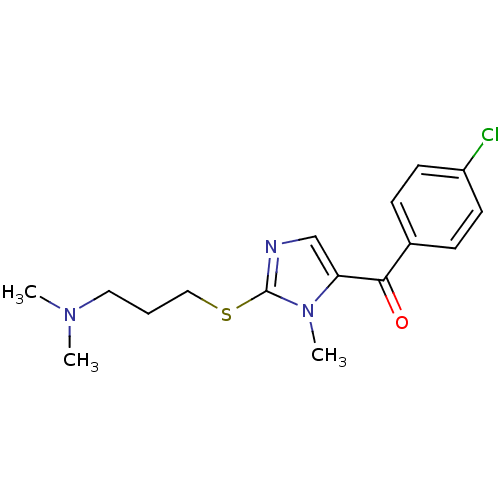
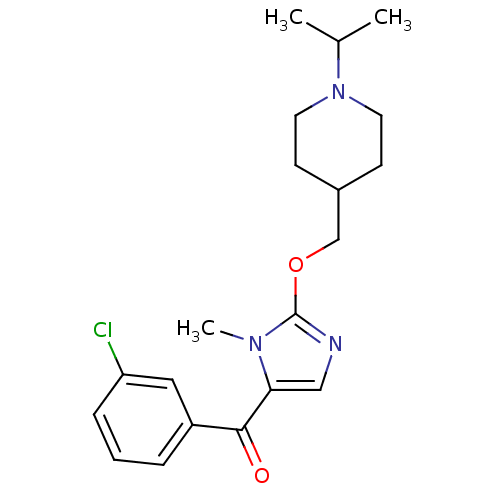
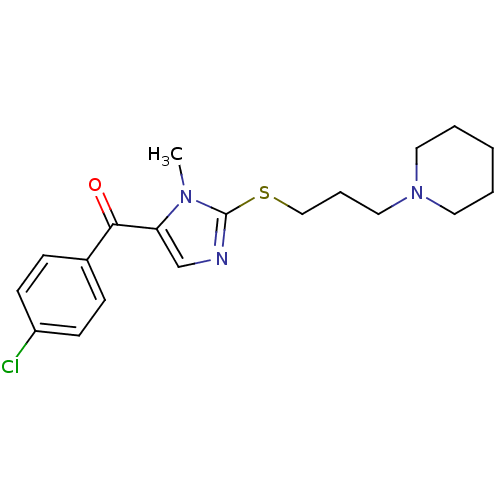
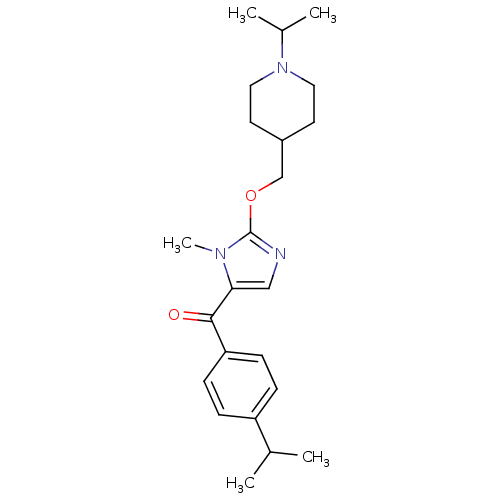
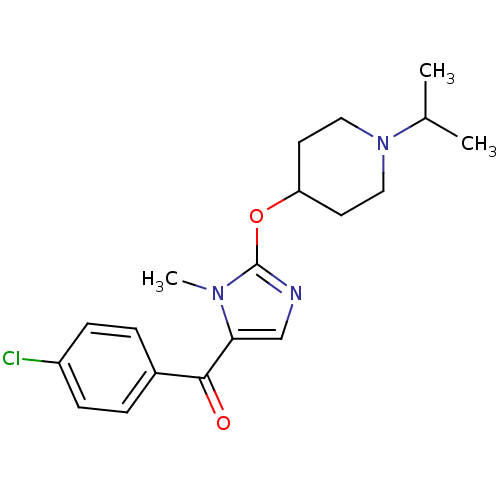
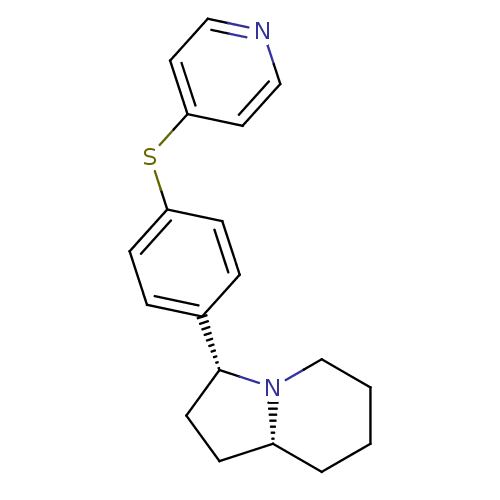
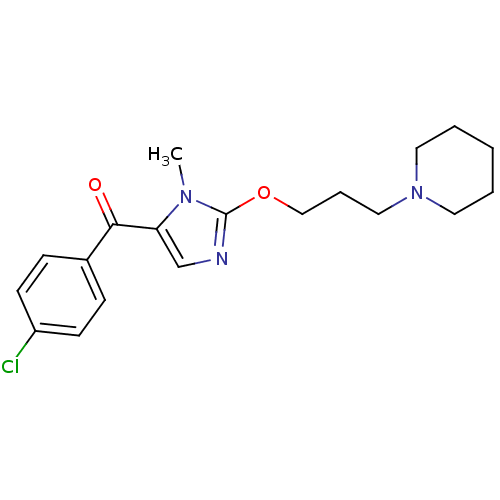
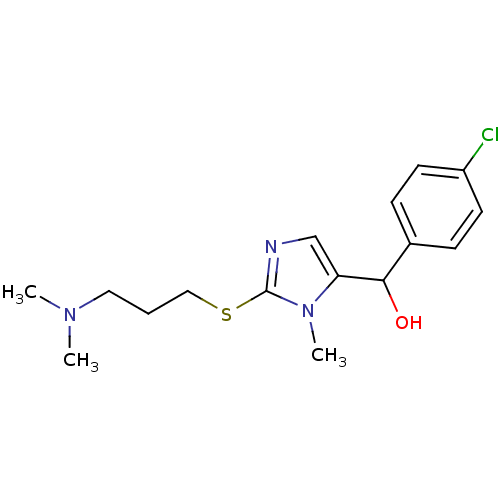
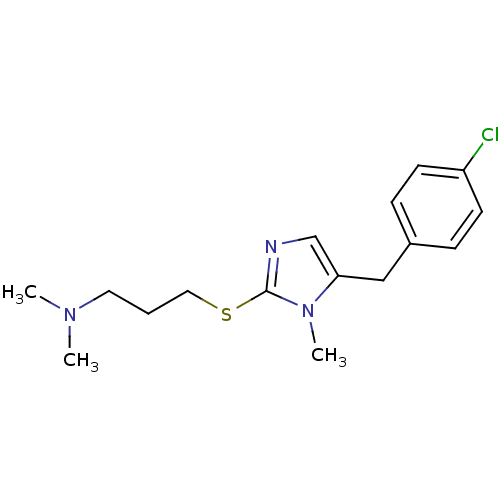
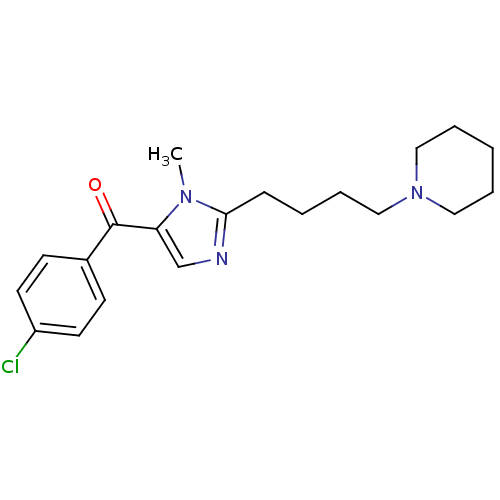



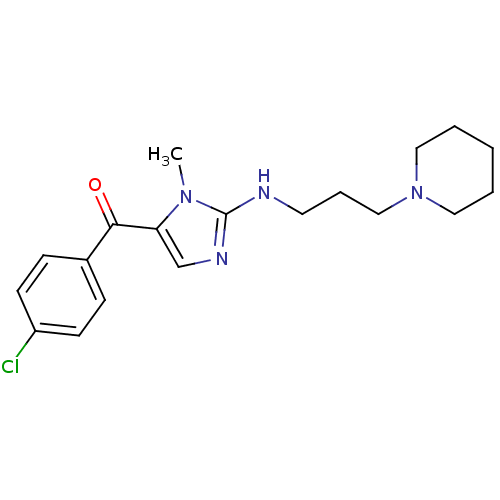
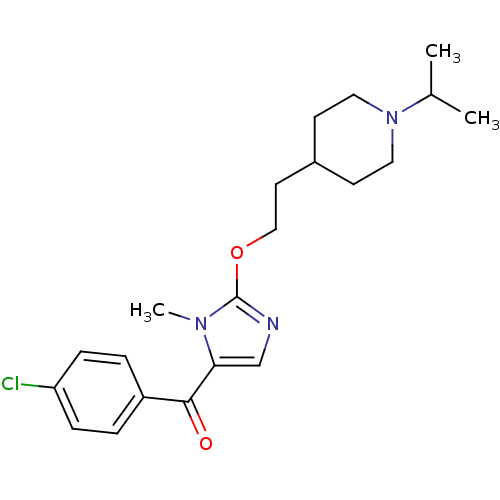
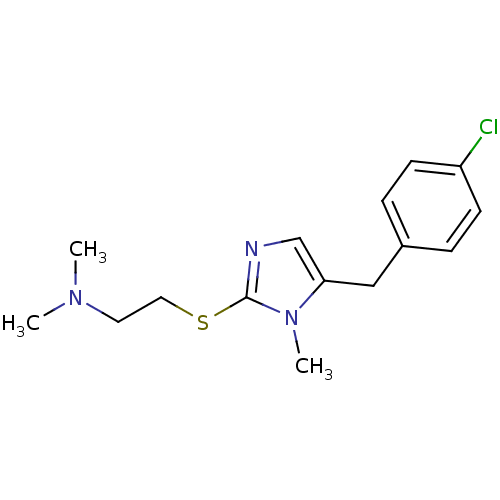
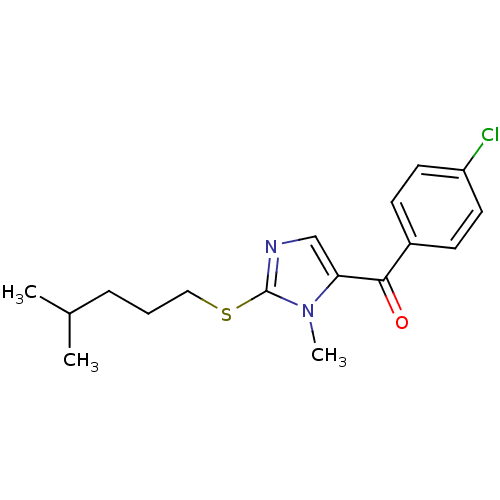
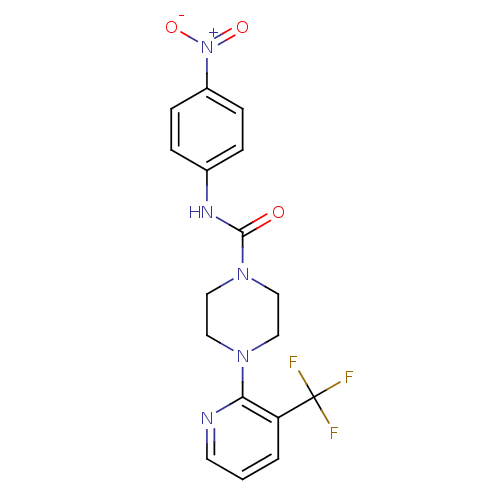
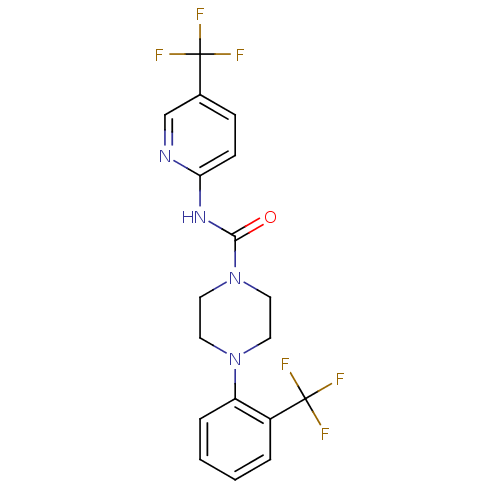
 3D Structure (crystal)
3D Structure (crystal)