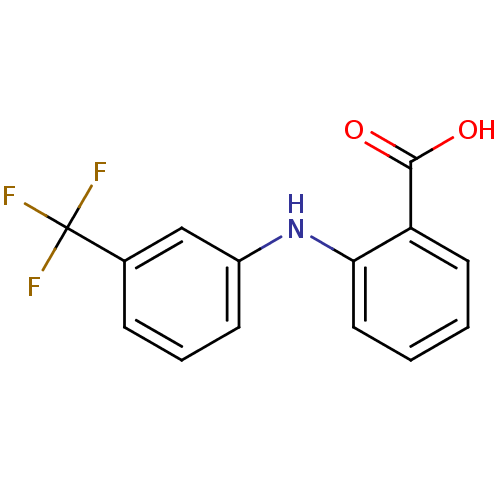
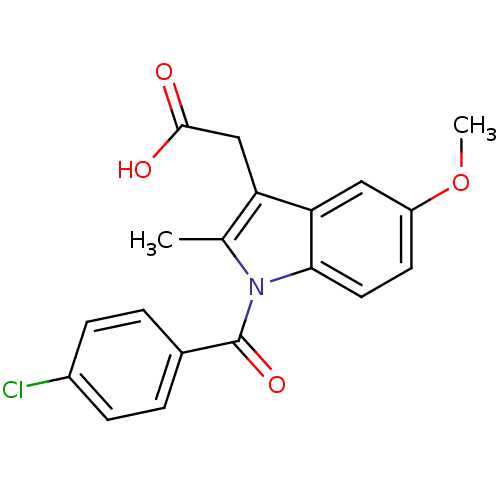
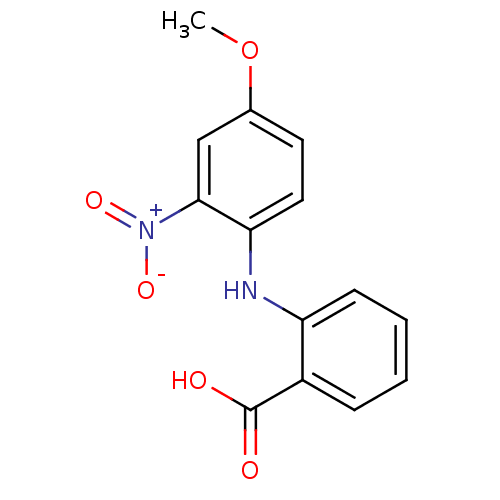
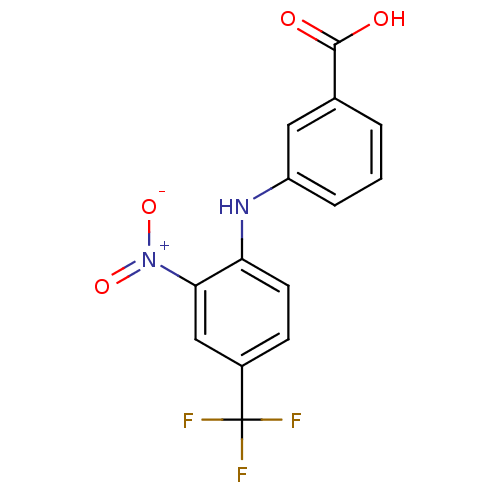
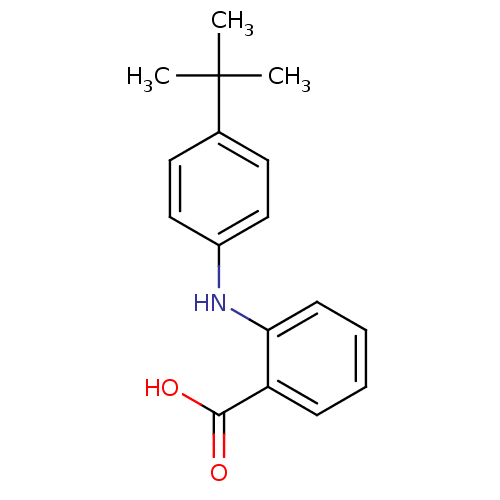
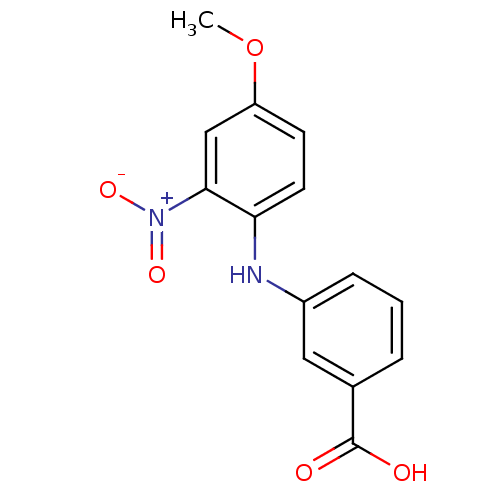
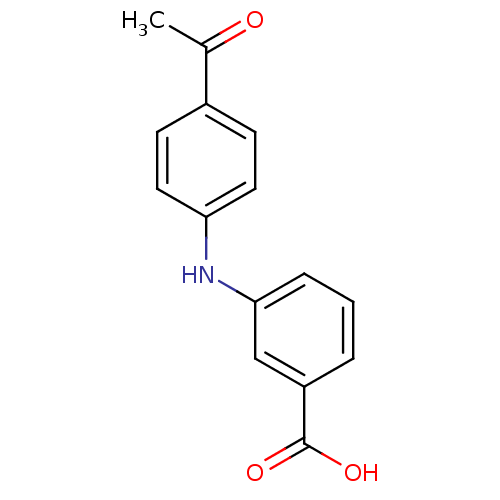
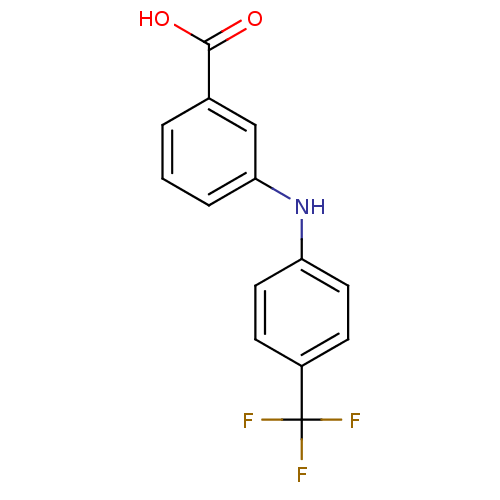
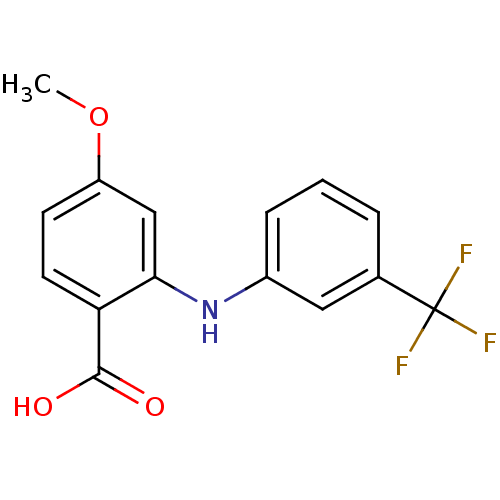
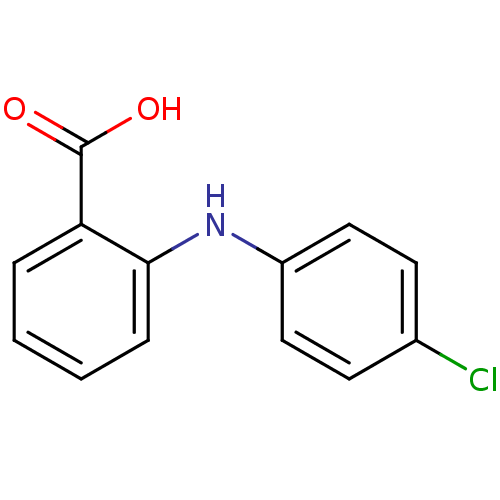
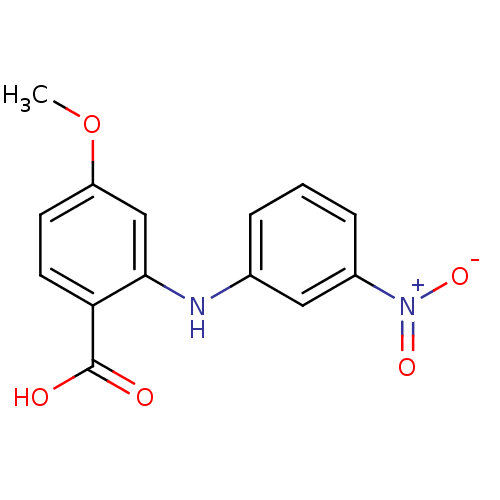
Affinity DataIC50: 0.0900nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.95nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.15nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.51nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 12.6nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 14.5nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of COX2 expressed in baculovirus infected SF-21 cells assessed as formation of PGH2 from PGG2 using arachidonic acid as substrate preincub...More data for this Ligand-Target Pair
Affinity DataIC50: 17.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Vanderbilt University School Of Medicine
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of COX1 (unknown origin)-mediated oxidation of N,N,N,Ntetramethyl-1,4-phenylenediamine using arachidonic acid as substrate by colorimetric...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant AKR1B1 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 35.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 48.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 49.6nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 49.8nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Vanderbilt University School Of Medicine
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of ovine COX1 by discontinuous radioactive TLC assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50.1nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 53.5nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 54.5nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 62nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 76.3nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair

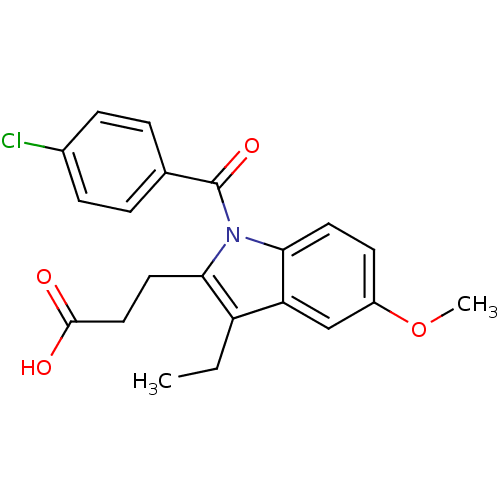
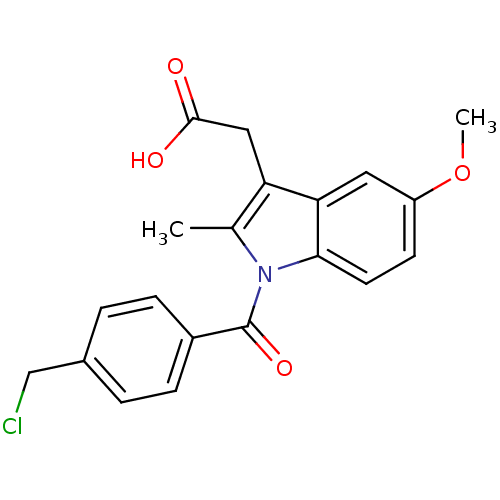

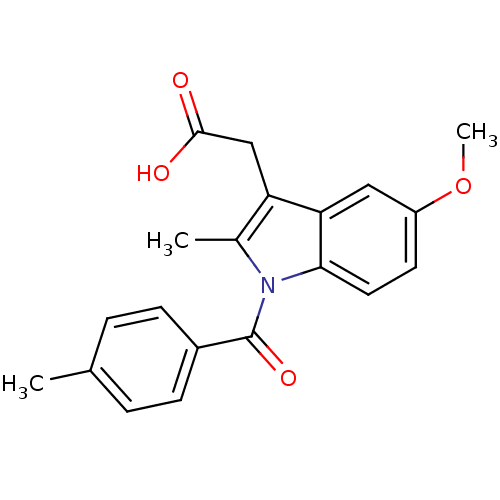
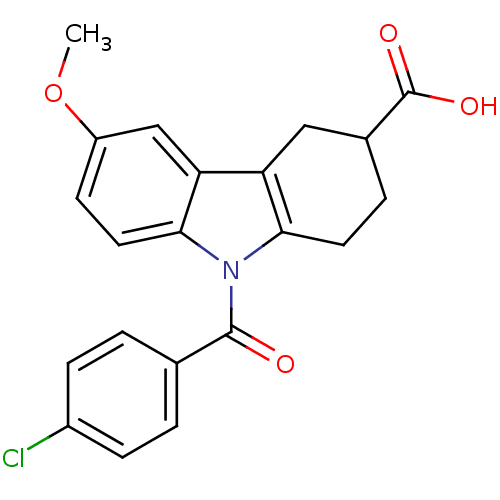
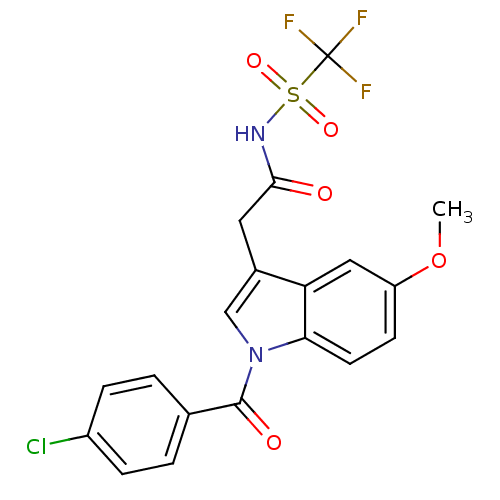
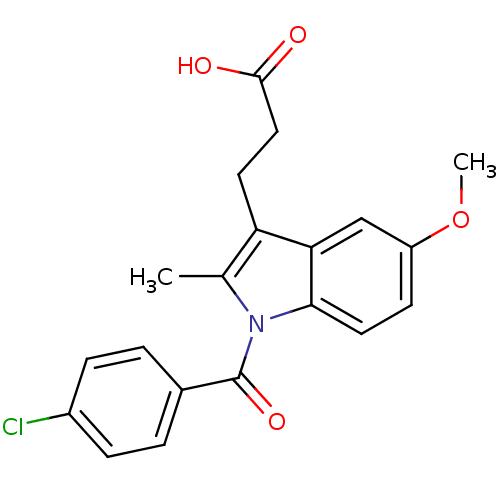
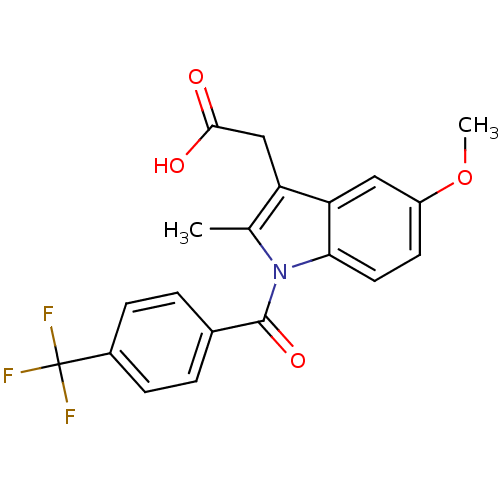
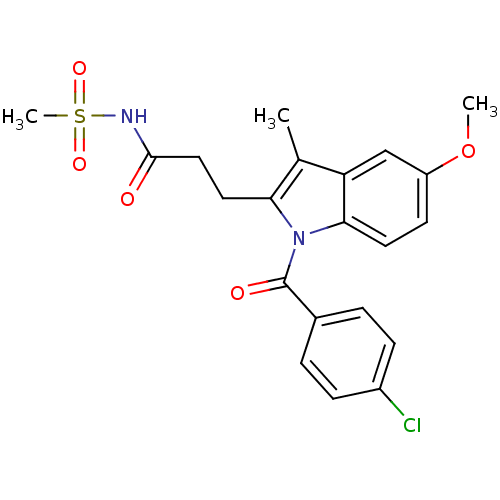
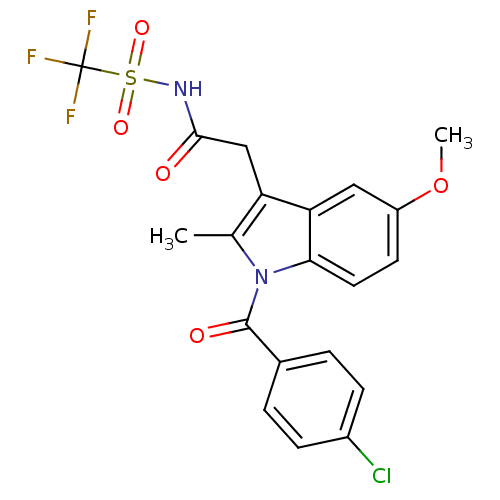
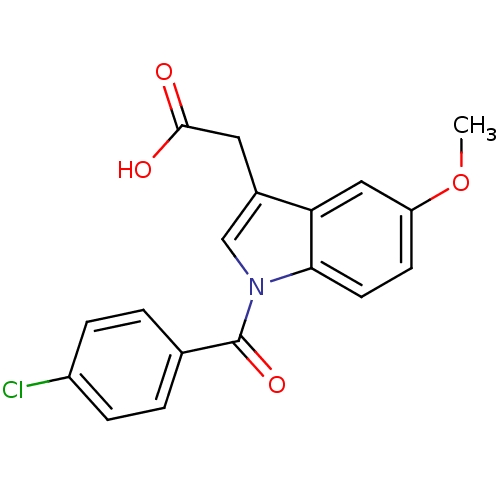
 3D Structure (crystal)
3D Structure (crystal)