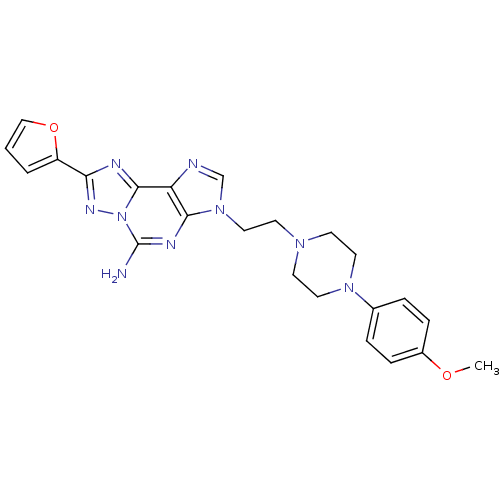
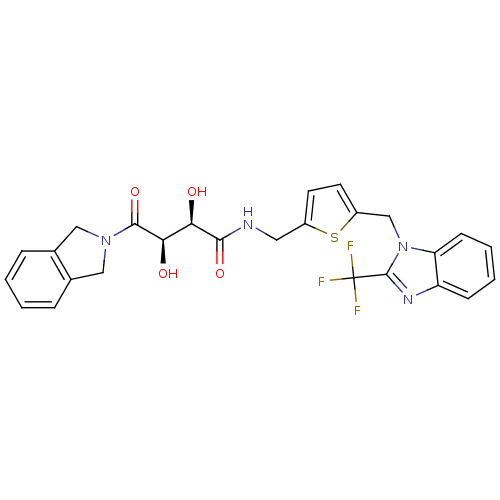
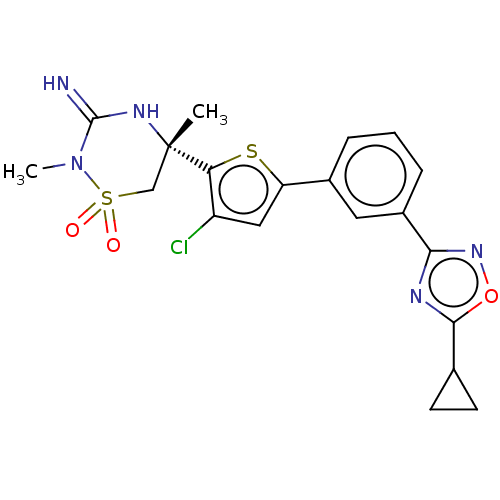
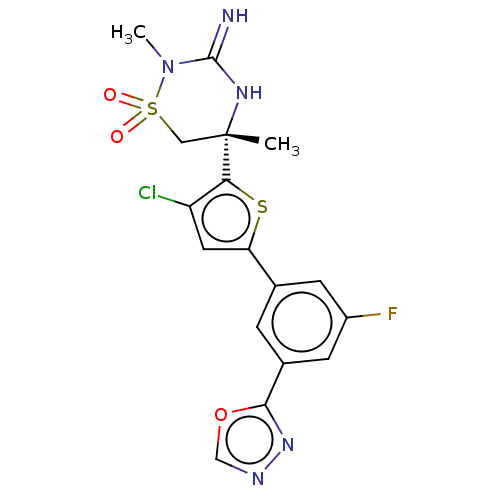
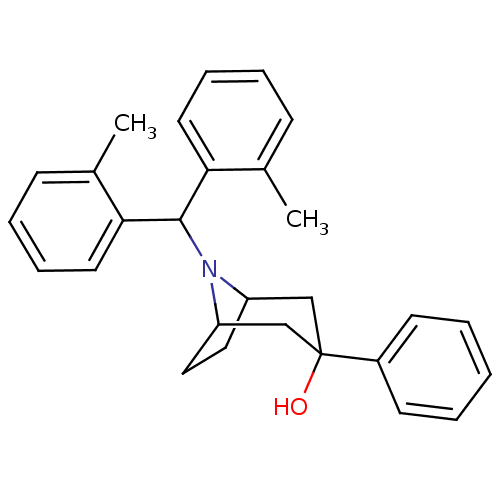
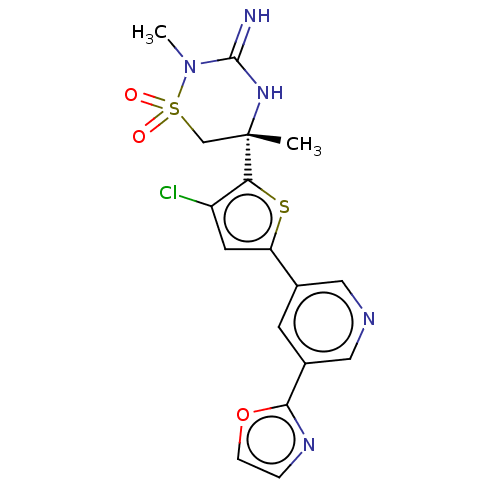
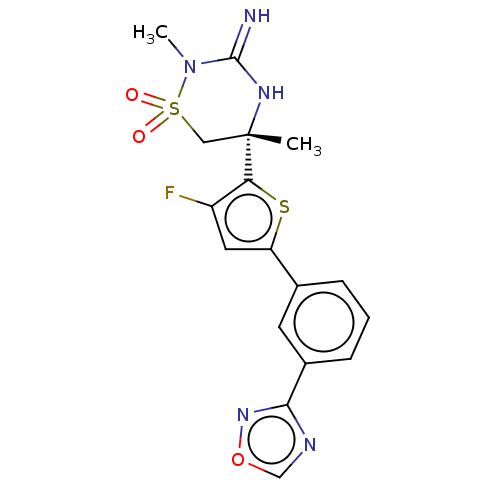
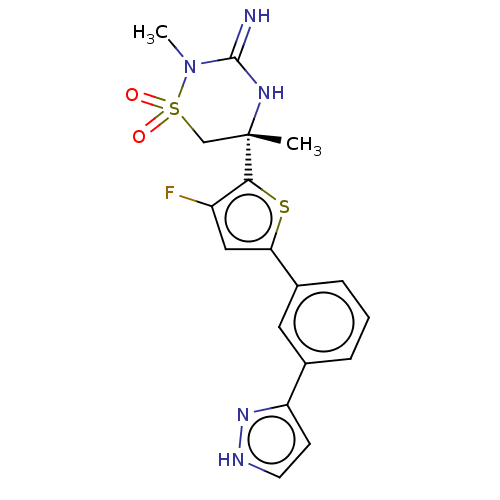
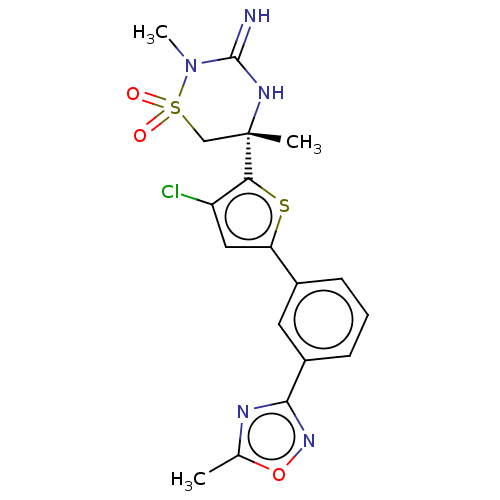
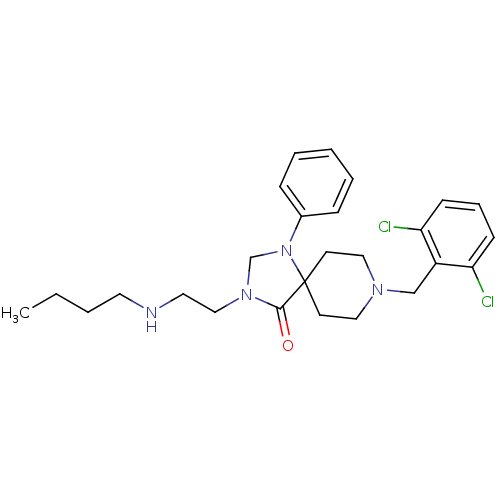
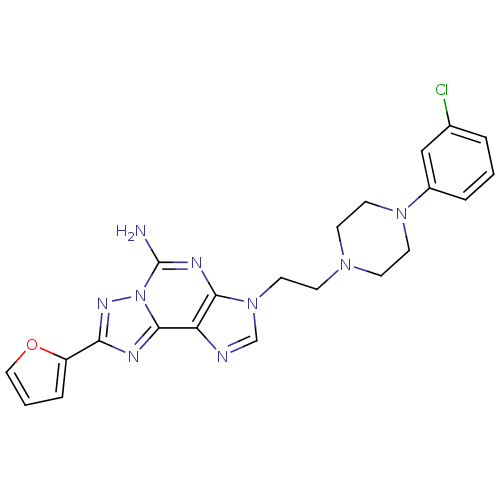
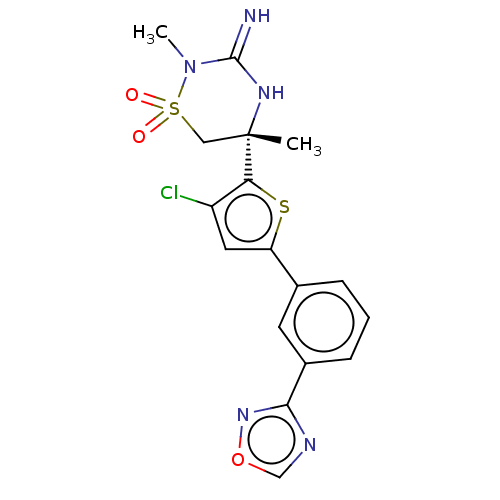
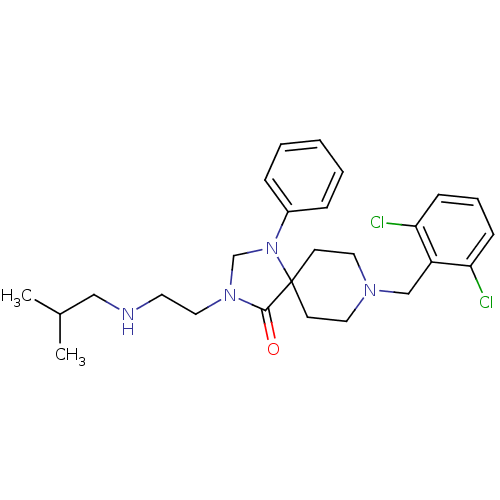
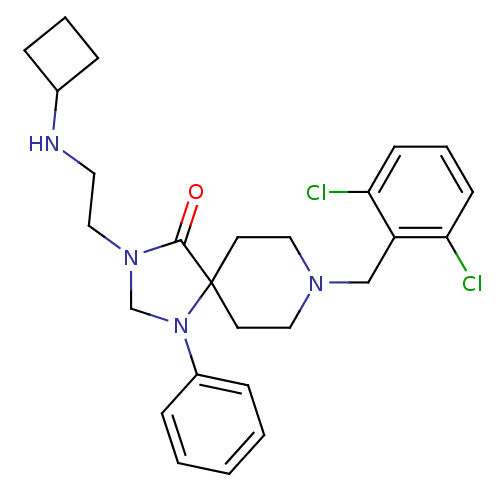
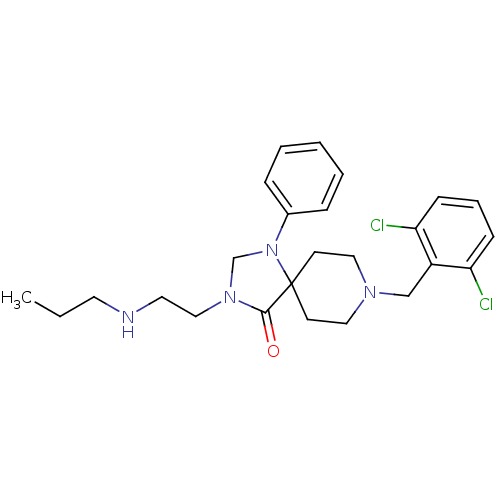
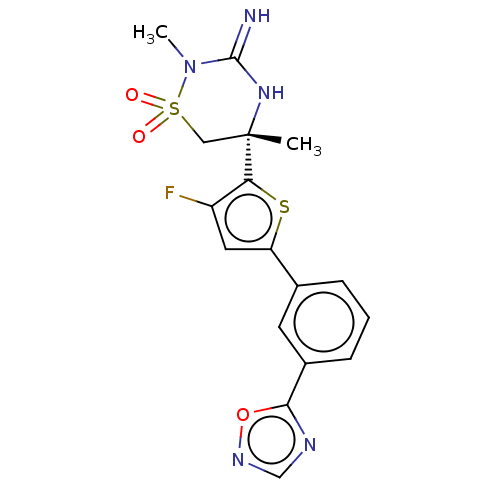
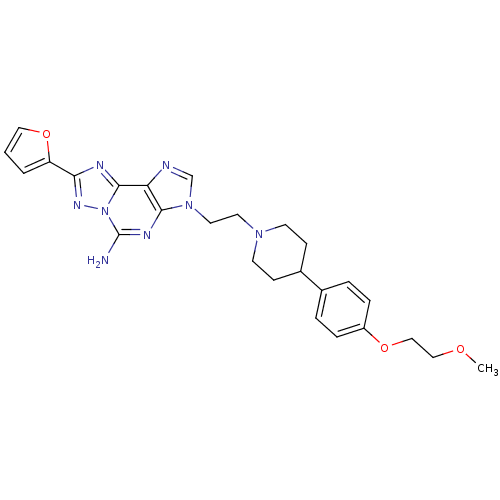
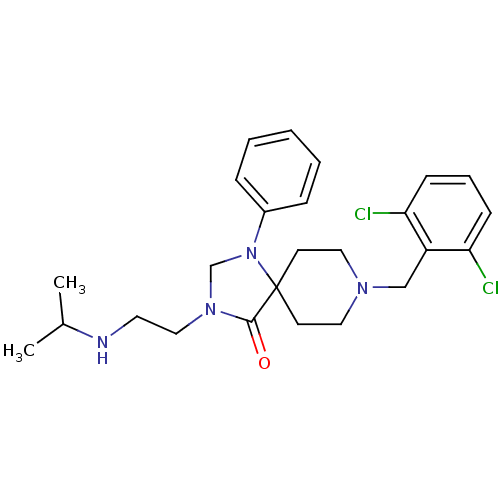
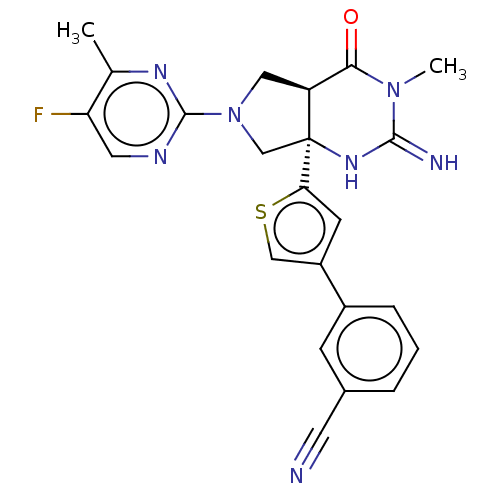
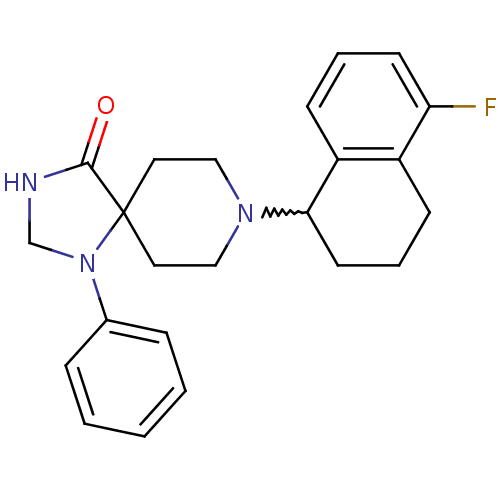
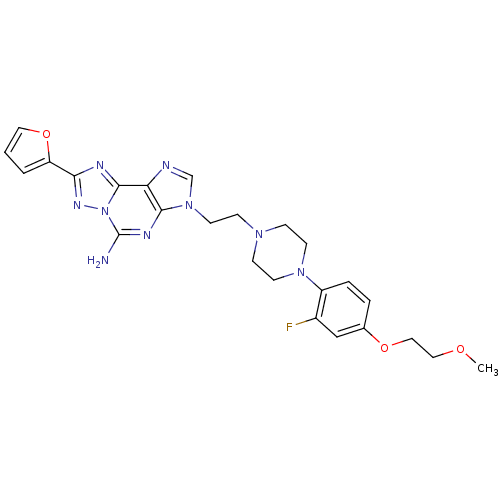
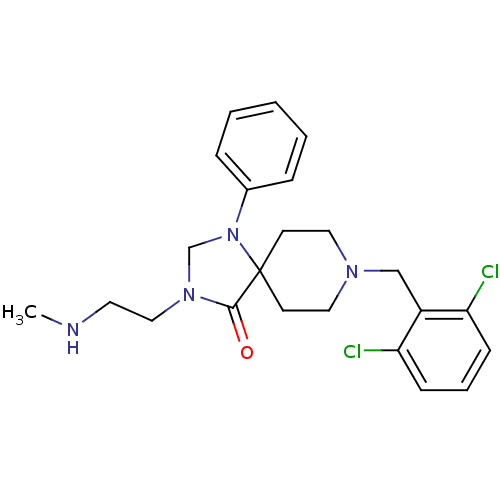
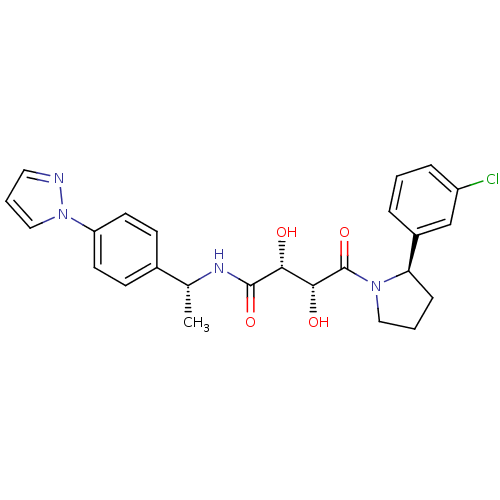
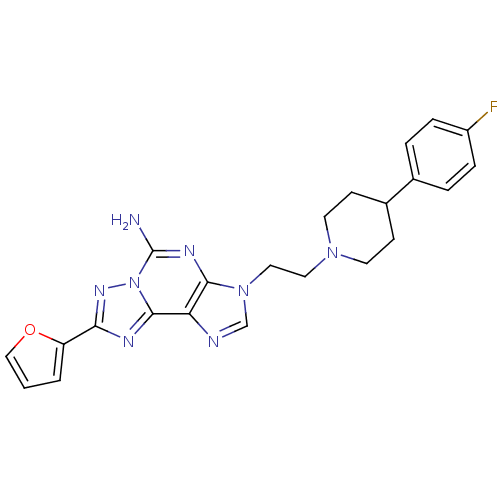
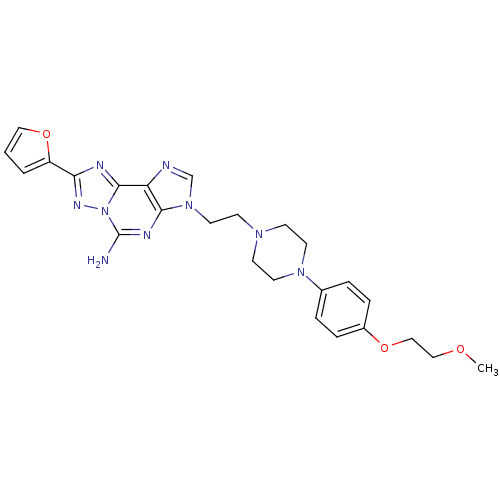
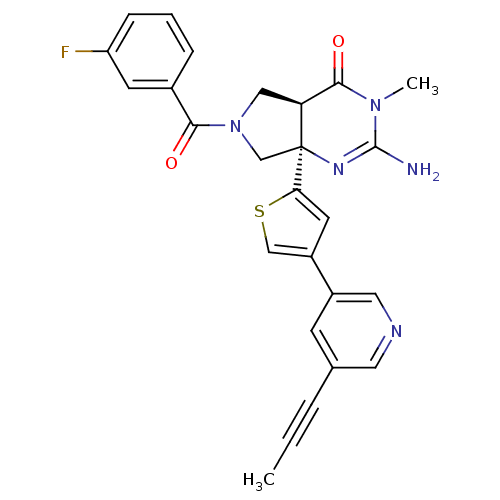
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
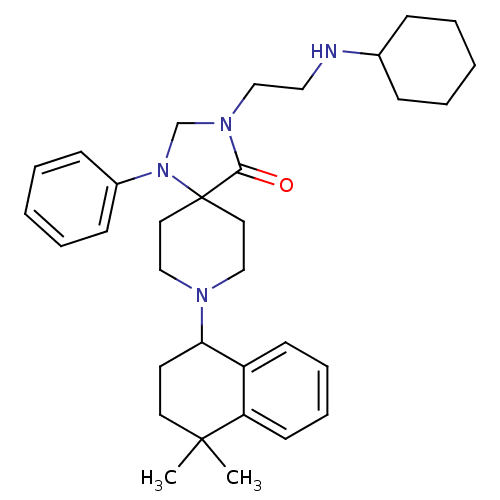
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.240nM ΔG°: -55.8kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.280nM ΔG°: -55.4kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membraneMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human cloned NOP receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nM ΔG°: -55.2kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.340nM ΔG°: -54.9kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.350nM ΔG°: -54.9kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.350nM ΔG°: -54.9kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.410nM ΔG°: -54.5kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 5.0 T: 2°CAssay Description:Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nM ΔG°: -53.4kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of recombinant human BACE1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of recombinant human BACE1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.730nM ΔG°: -53.0kJ/molepH: 5.0 T: 2°CAssay Description:Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0....More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membraneMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.870nM ΔG°: -52.6kJ/molepH: 5.0 T: 2°CAssay Description:Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membraneMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Antagonist activity at human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f...More data for this Ligand-Target Pair