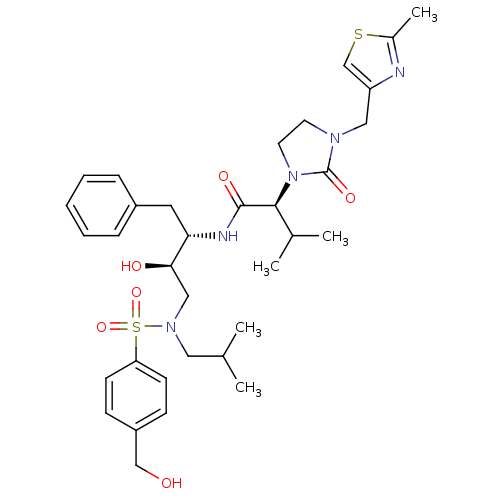
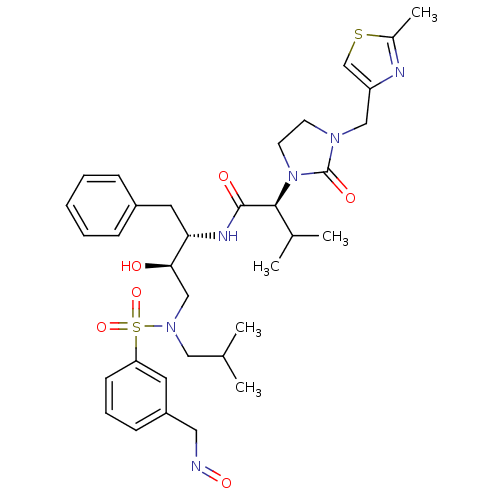
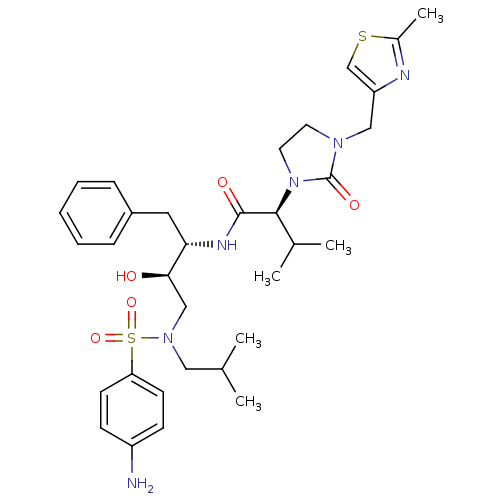
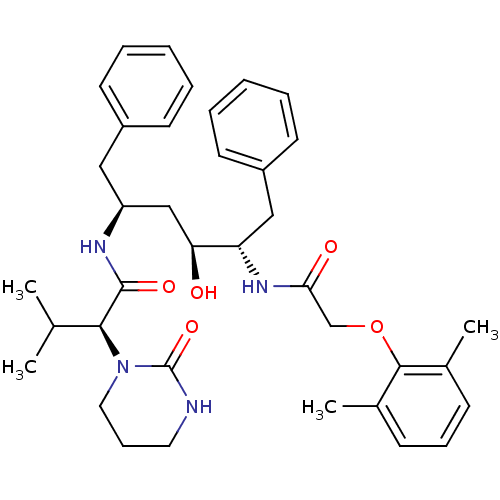
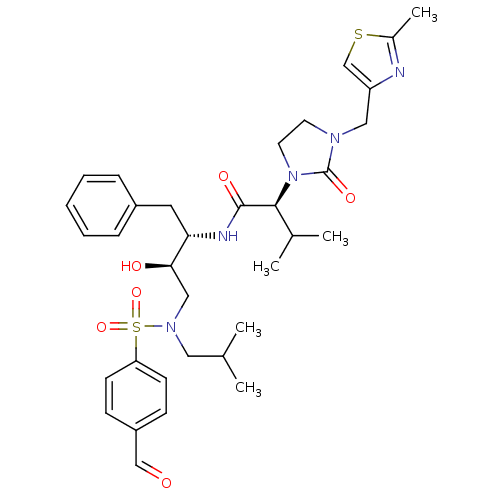
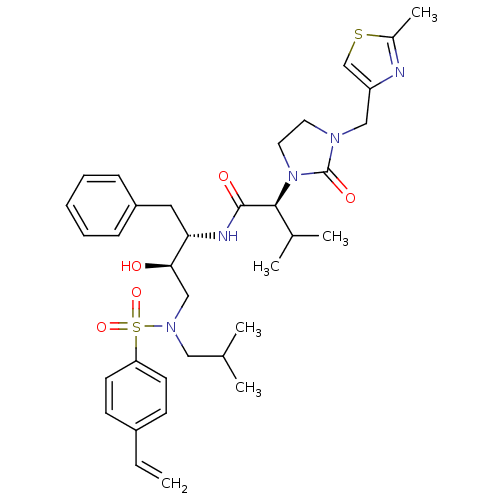
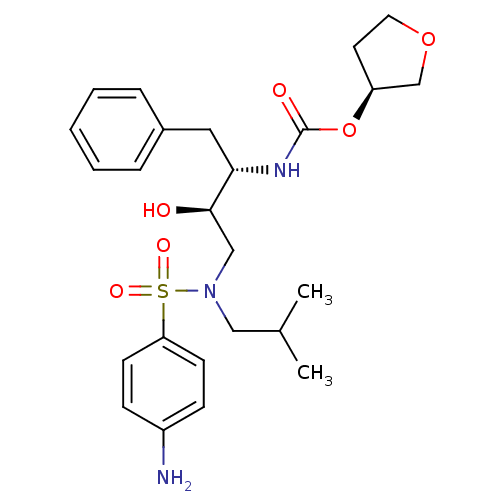
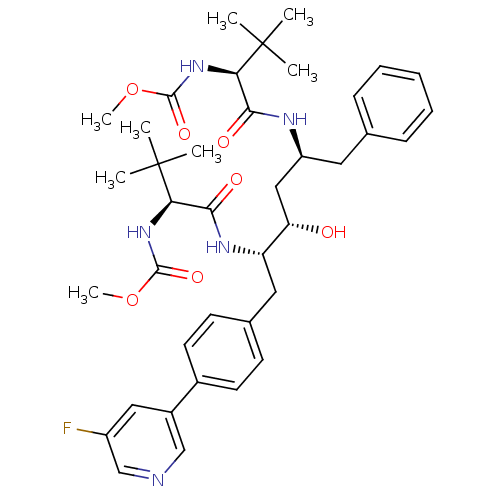
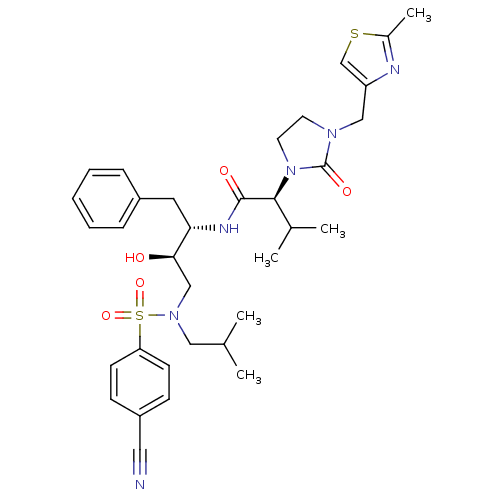
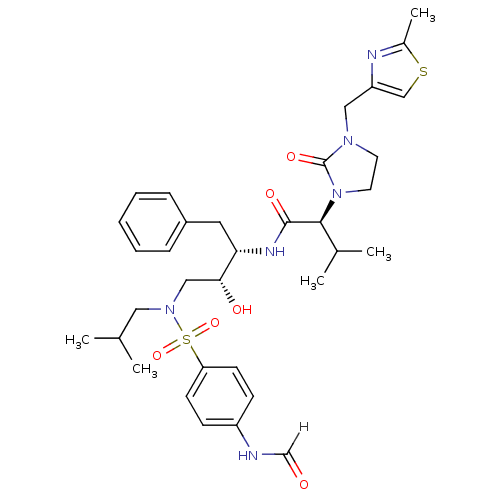
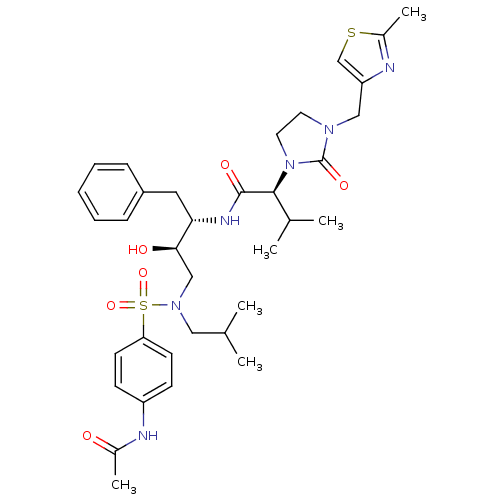
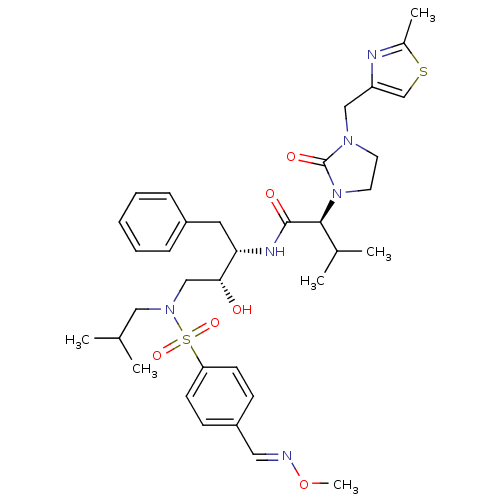
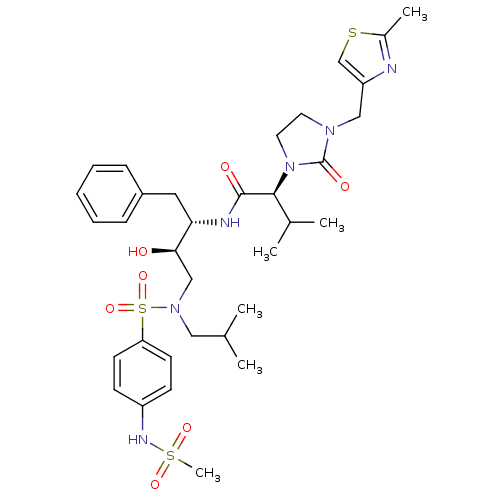
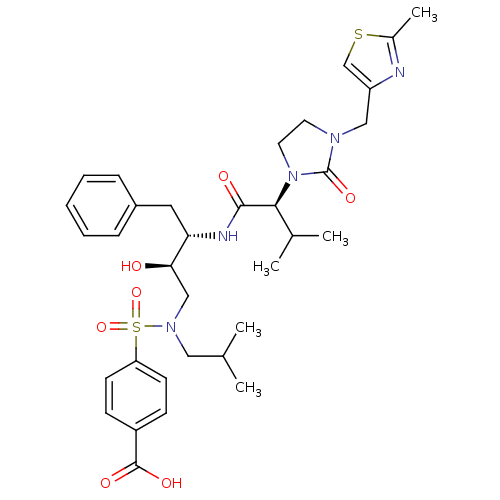
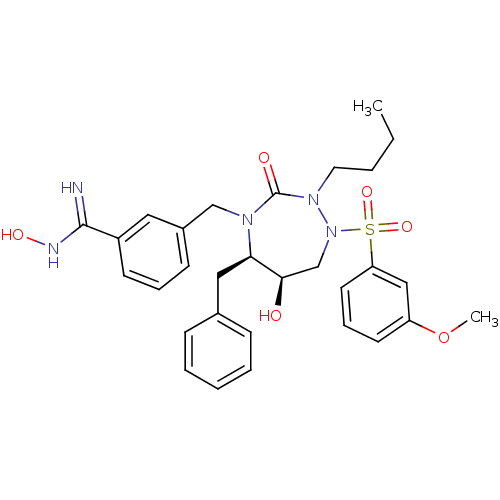
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0100nMAssay Description:Tested for inhibition of HIV proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0700nMAssay Description:Tested for inhibition of HIV proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.160nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.25nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.470nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 1.37nMAssay Description:Tested for inhibition of HIV proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 5.80nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Inhibition of cathepsin D (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of cathepsin D (unknown origin)More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 5nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 16nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 16nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 31nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 35nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 39nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 45nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 51nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 67nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 101nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 109nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 143nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 148nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 168nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 254nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 312nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 653nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.19E+3nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of CYP1A2 (unknown origin) assessed as phenacetin O-deethylationMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin) assessed as S-mephenytion hydroxylationMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibitory activity of the compound against HIV-1 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin) assessed as dextromethorphan O-demethylationMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin) assessed as tolbutamide hydroxylationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin) assessed as S-mephenytion hydroxylationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin) assessed as tolbutamide hydroxylationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) assessed as phenacetin O-deethylationMore data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.00E+4nMpH: 4.5 T: 2°CAssay Description:HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) assessed as dextromethorphan O-demethylationMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 30nMAssay Description:Antiviral activity against HIV protease in absence of human serumMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Antiviral activity against HIV proteaseMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:A cytopathetic effect (CPE) protection assay was performed to determine the ability of a compound to protect the cells from viral infection and thus ...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:A cytopathetic effect (CPE) protection assay was performed to determine the ability of a compound to protect the cells from viral infection and thus ...More data for this Ligand-Target Pair







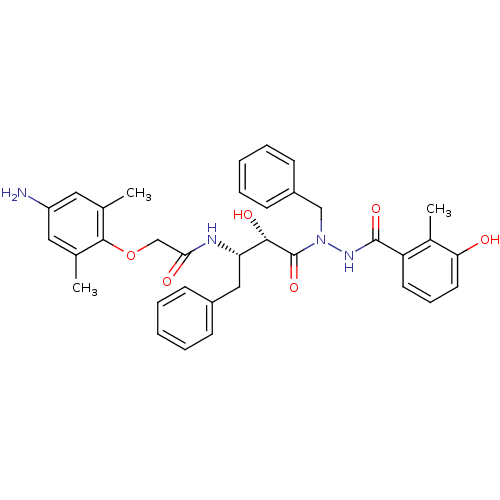

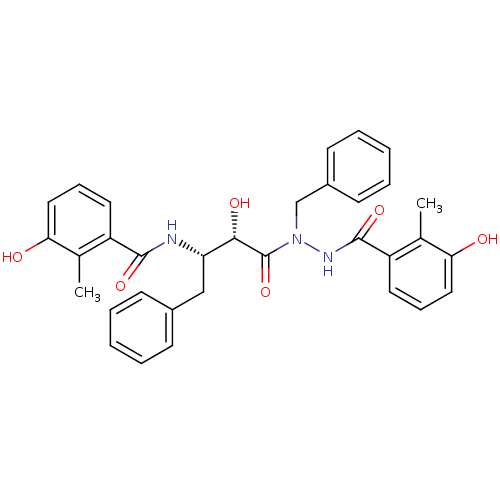
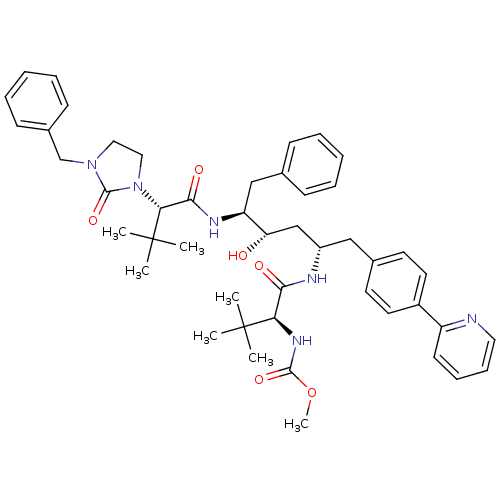
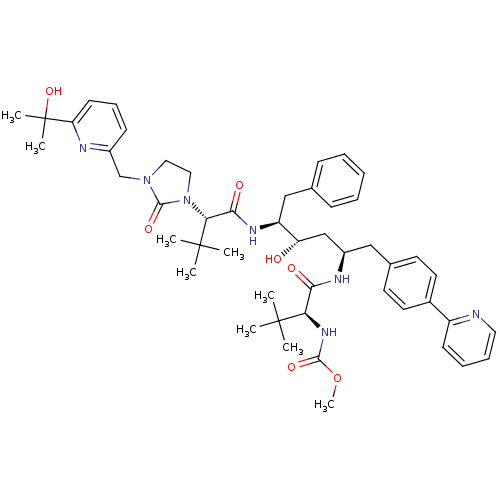

 3D Structure (crystal)
3D Structure (crystal)