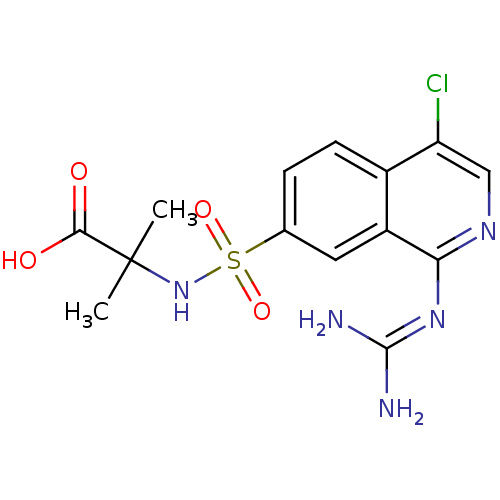
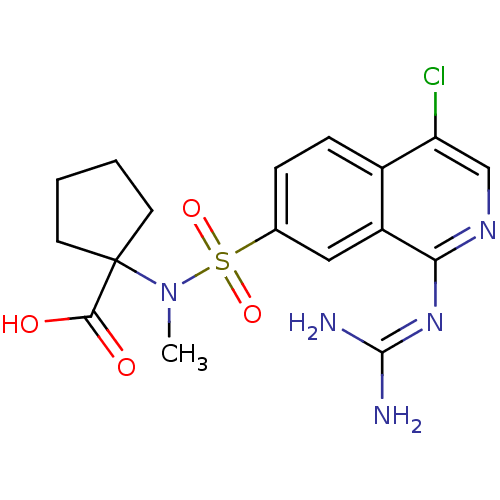
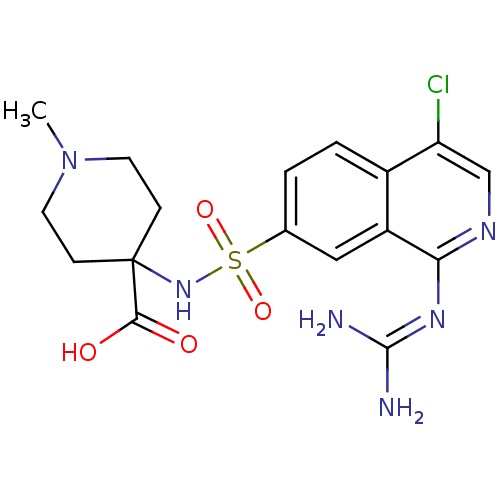
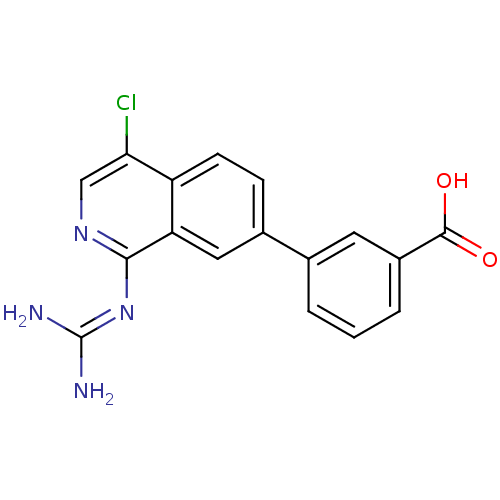
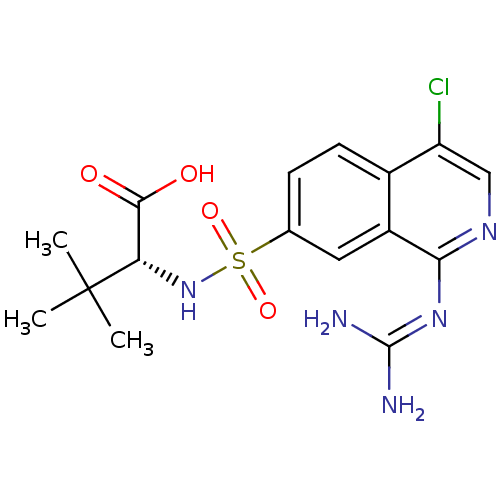
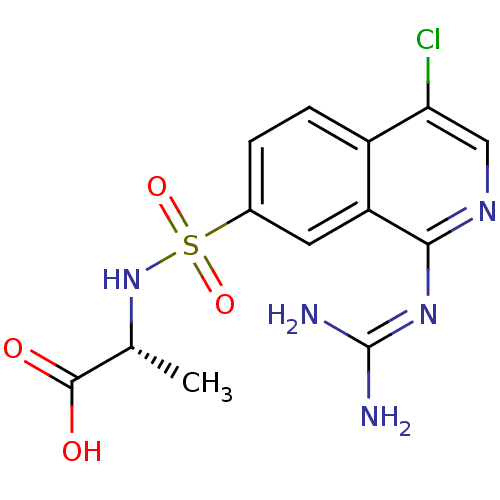
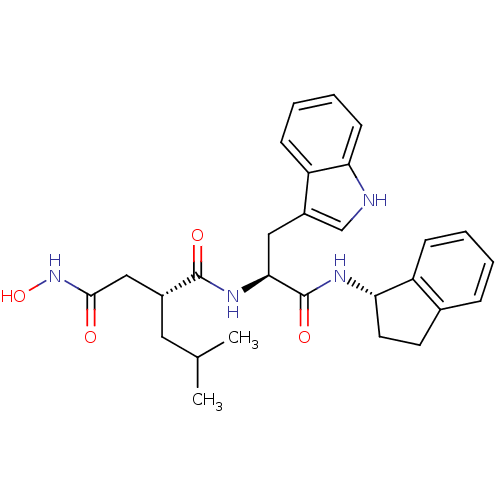
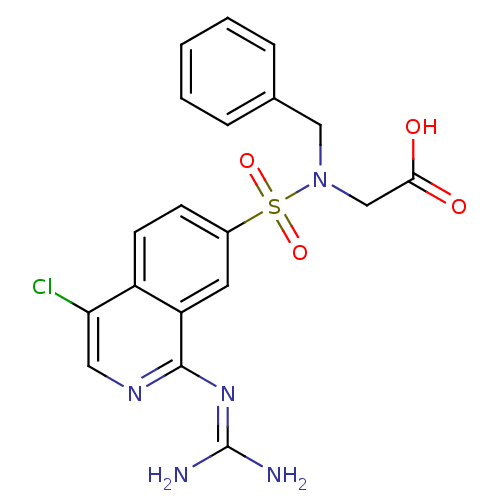
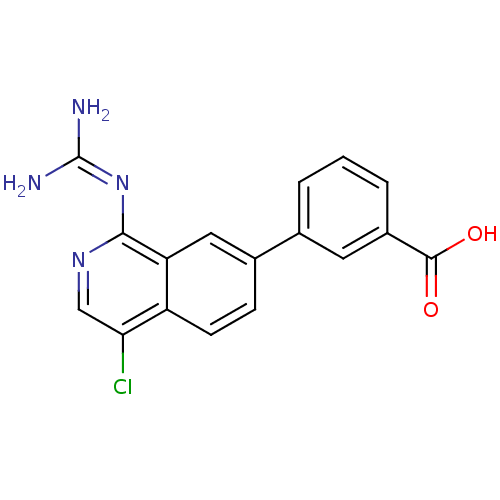
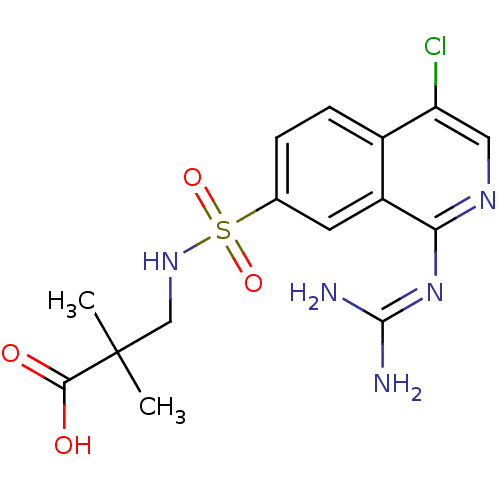
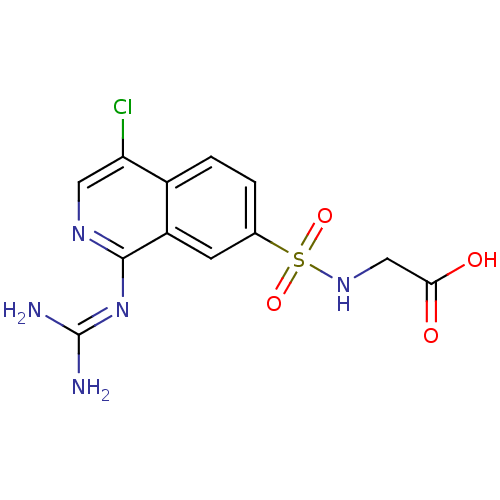
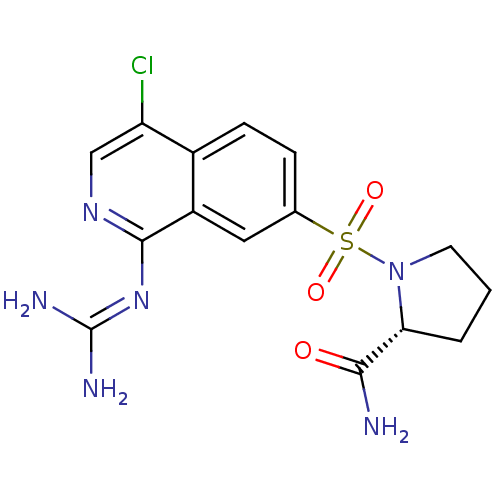
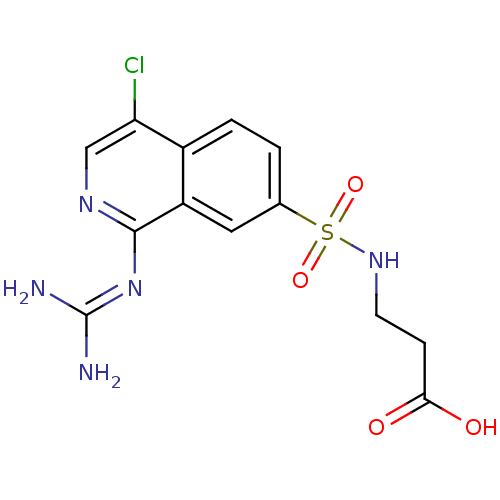
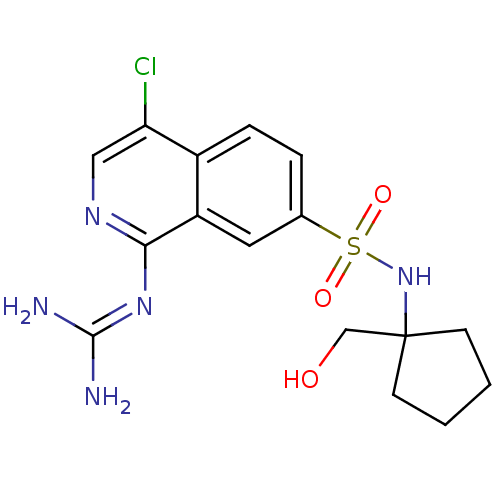
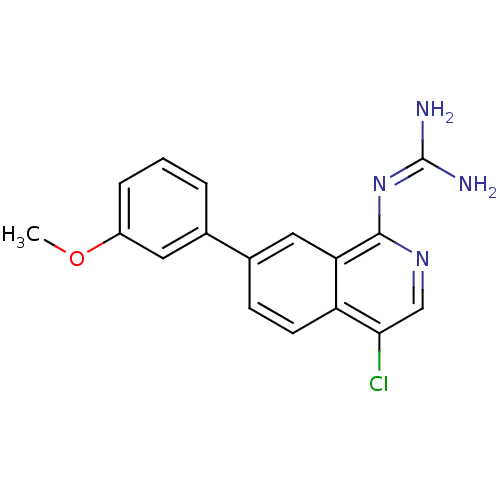
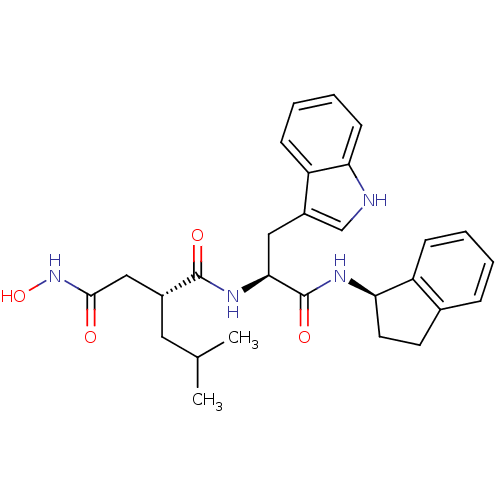
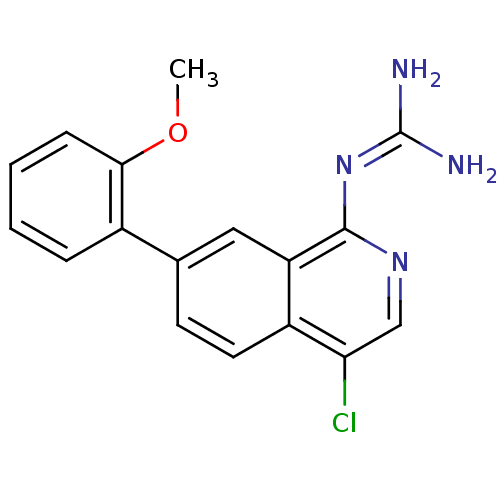
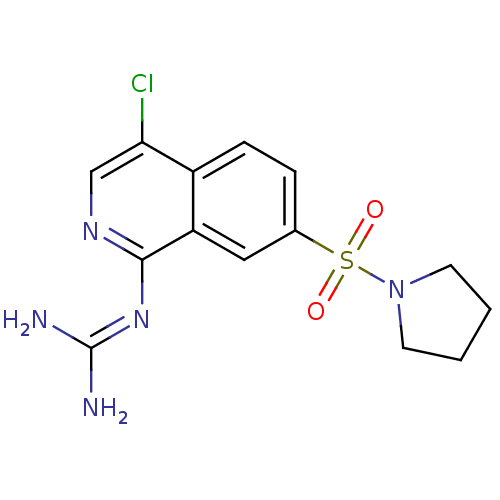
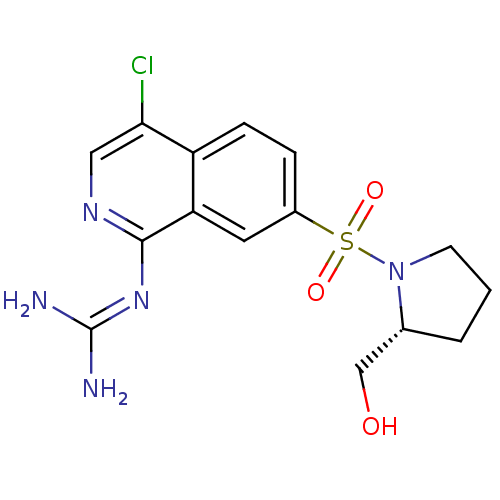
Affinity DataKi: 0.570nMAssay Description:Inhibition of Matrix metalloproteinase-2More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 5.80nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 17nM ΔG°: -46.1kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of Matrix metalloproteinase-3More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of Matrix metalloproteinase-2More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -43.7kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 48nM ΔG°: -43.5kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 58nM ΔG°: -43.0kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 59nM ΔG°: -42.9kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 67nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 71nM ΔG°: -42.4kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 83nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 86nM ΔG°: -42.0kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Inhibition of Matrix metalloproteinase-3More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of Matrix metalloproteinase-2More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 111nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 130nM ΔG°: -40.9kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 140nM ΔG°: -40.7kJ/molepH: 8.1 T: 2°CAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4...More data for this Ligand-Target Pair

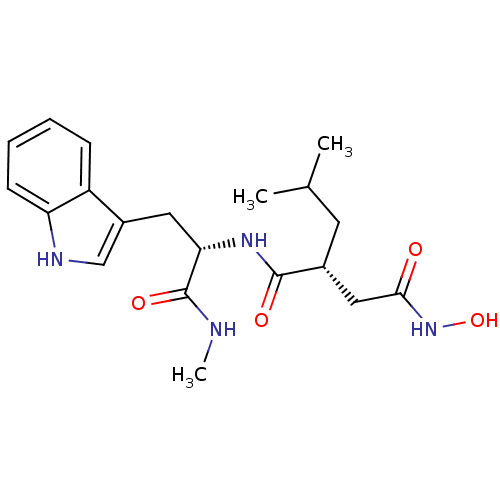




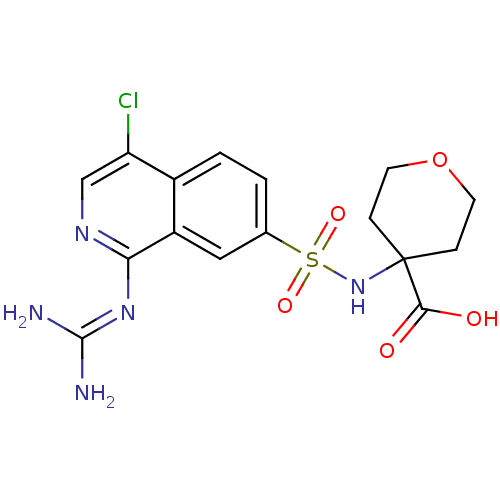
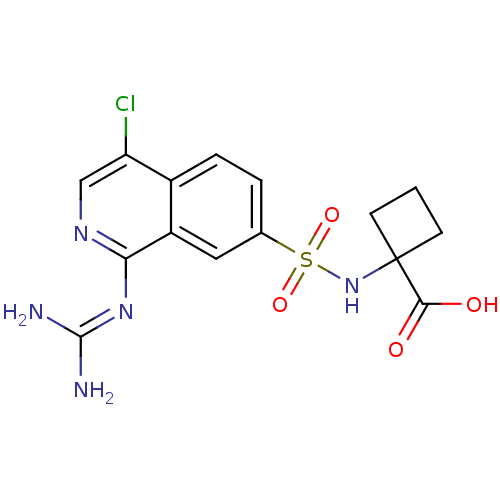


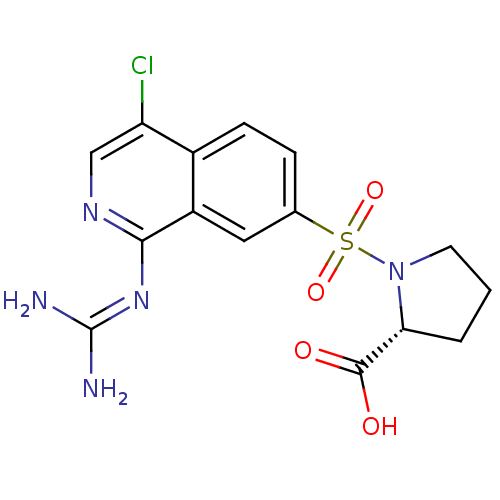
 3D Structure (crystal)
3D Structure (crystal)