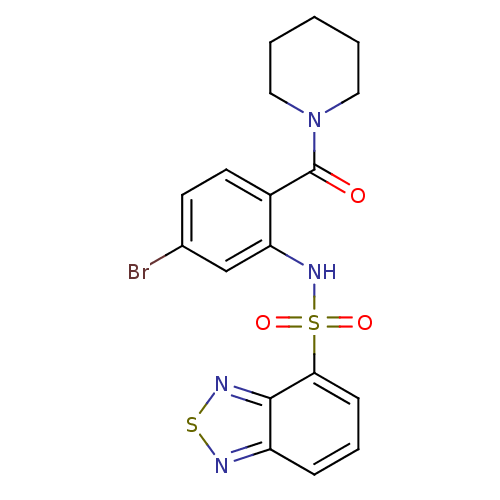
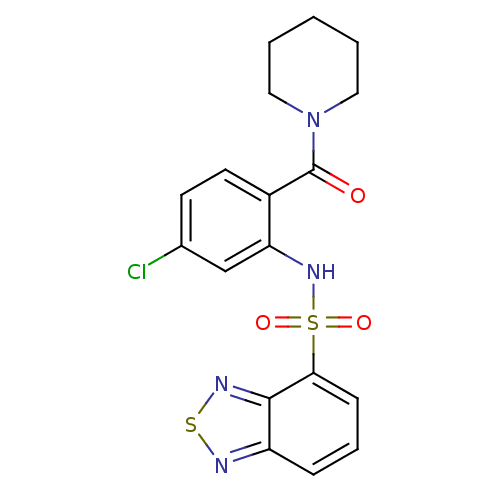
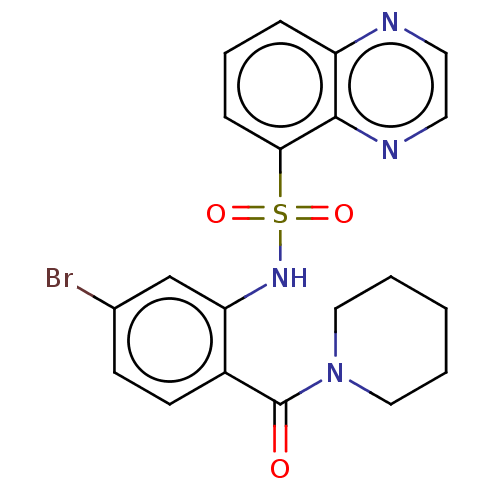
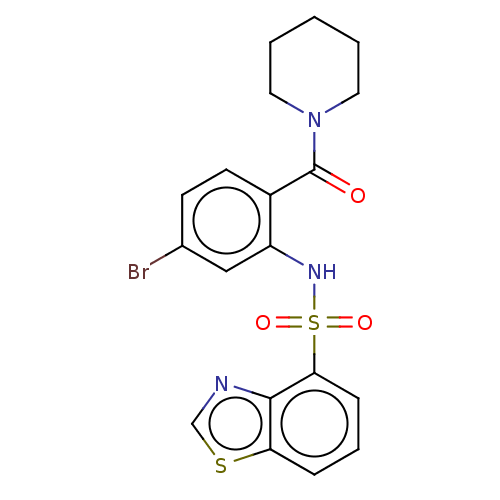
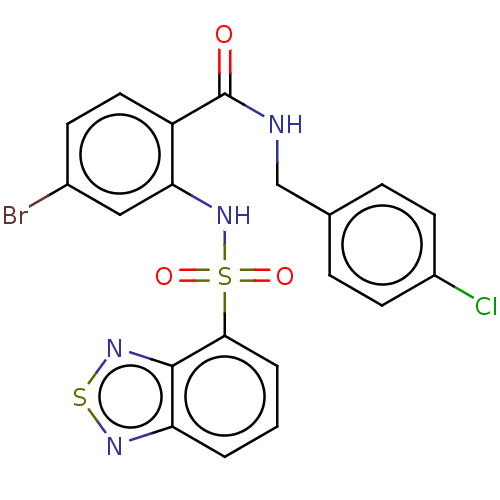
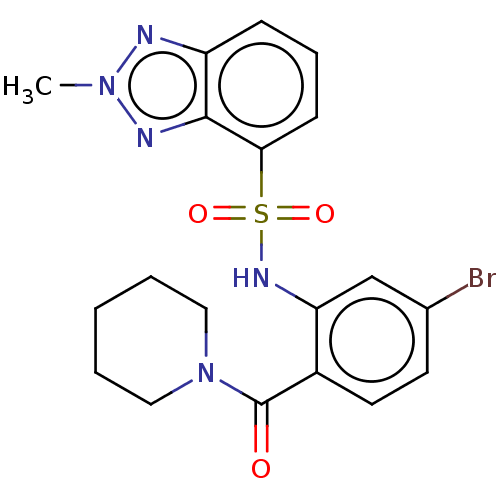
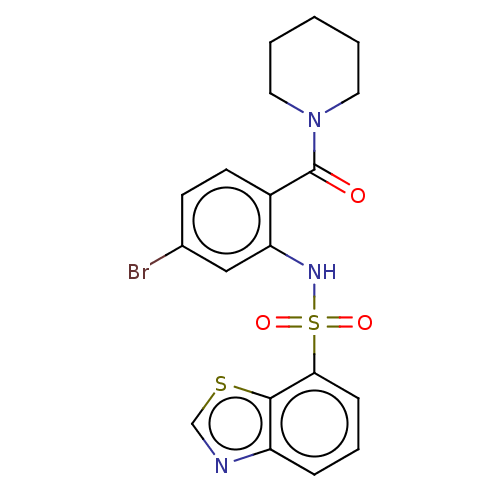
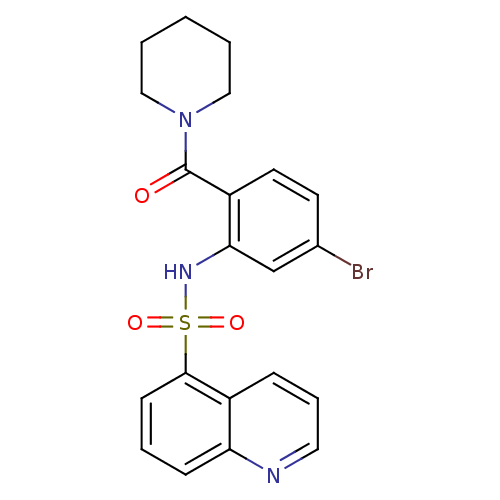
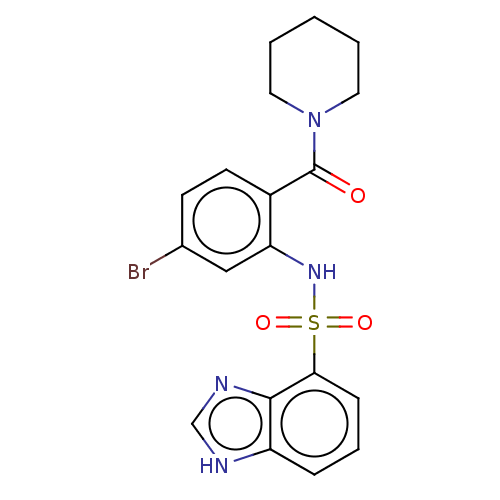
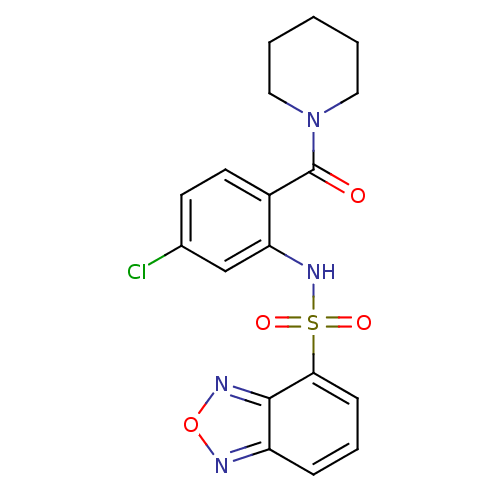
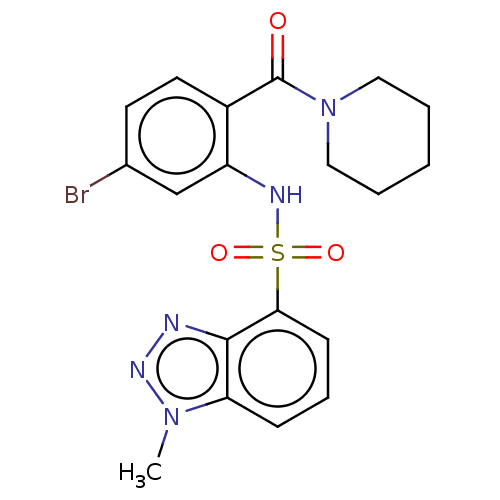
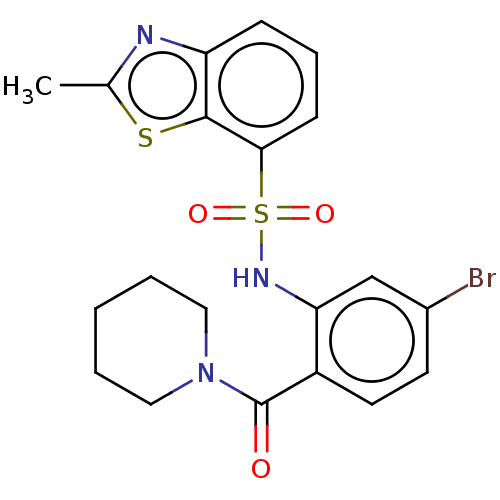
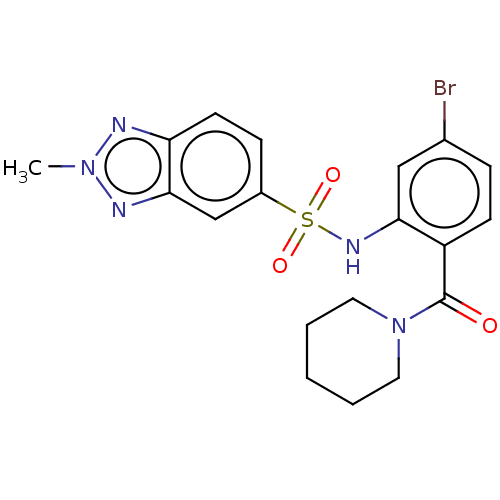
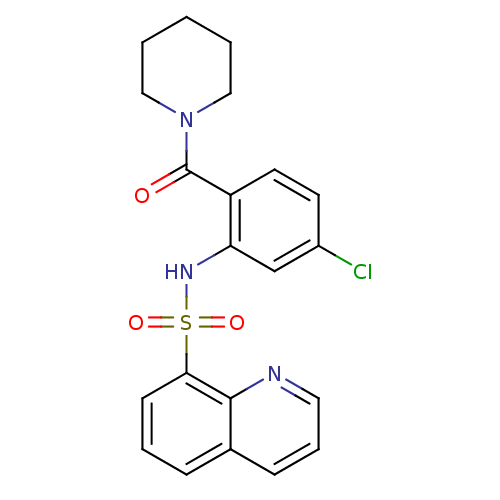
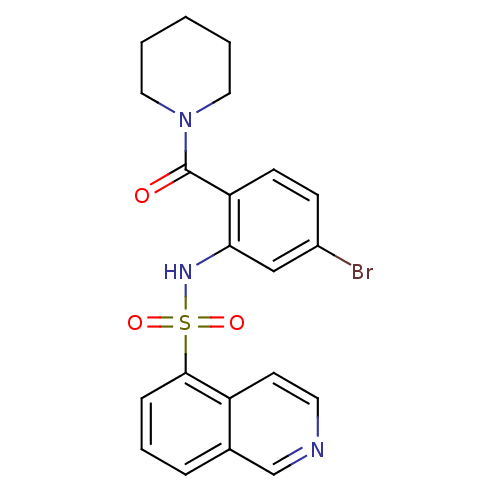
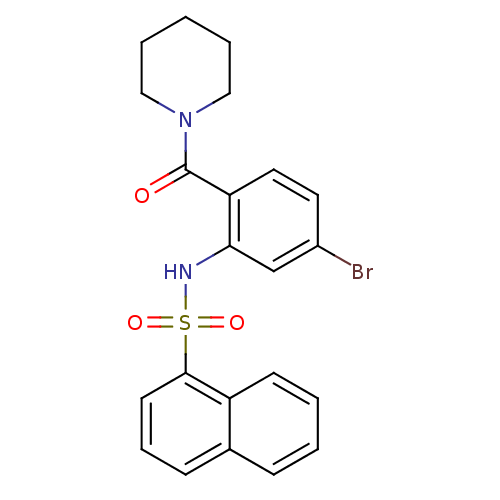
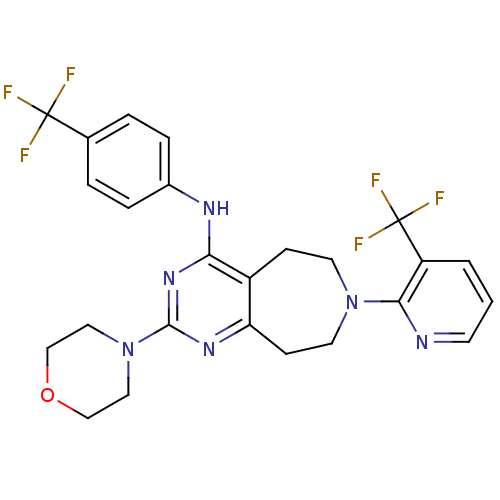
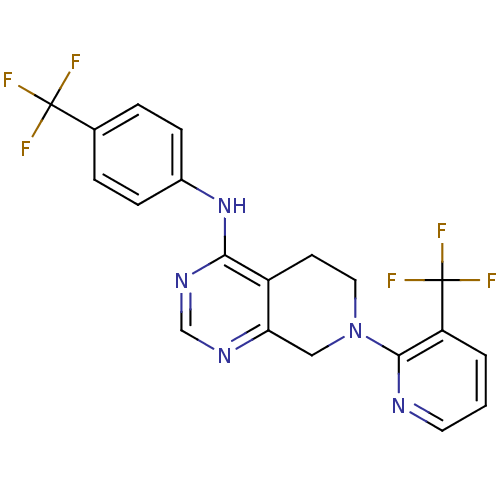
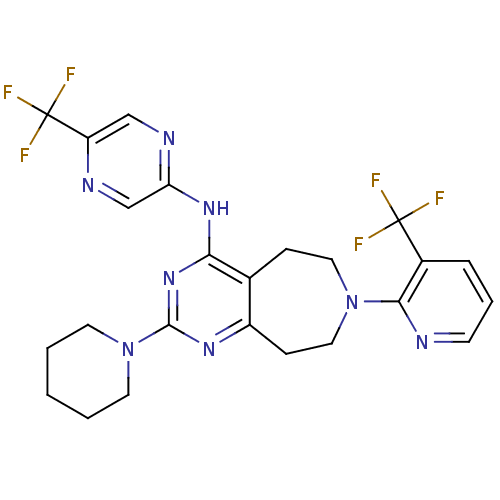
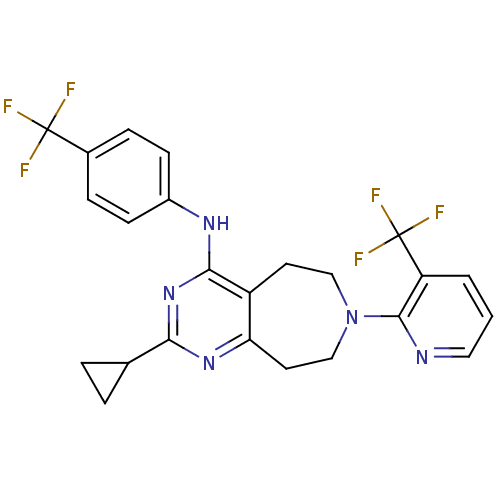
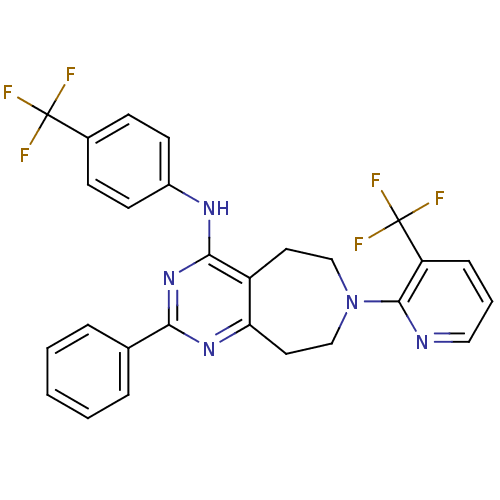
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Binding affinity to human recombinant TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Binding affinity to human recombinant TRPV1More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 79nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 501nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.59E+3nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.59E+3nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.16E+3nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.01E+3nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: <1.00E+4nMAssay Description:Displacement of [125I]CCK8S from human CCK1RMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity to rat TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assayMore data for this Ligand-Target Pair

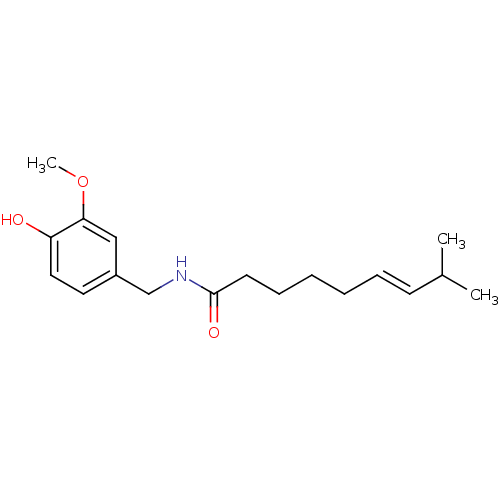
 3D Structure (crystal)
3D Structure (crystal)