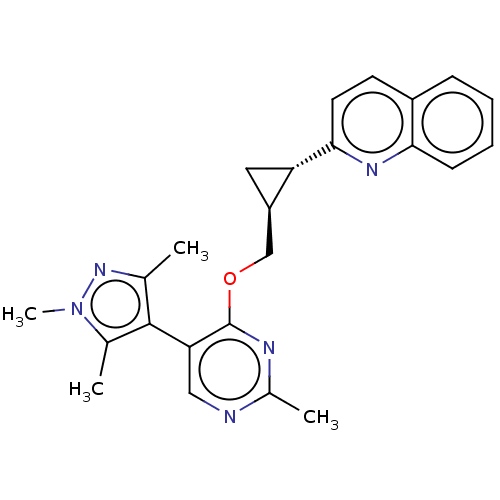
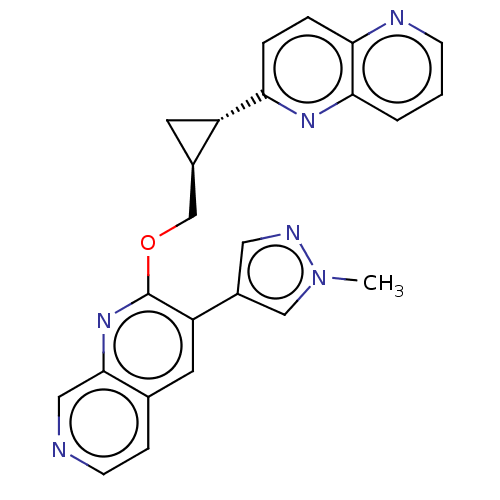
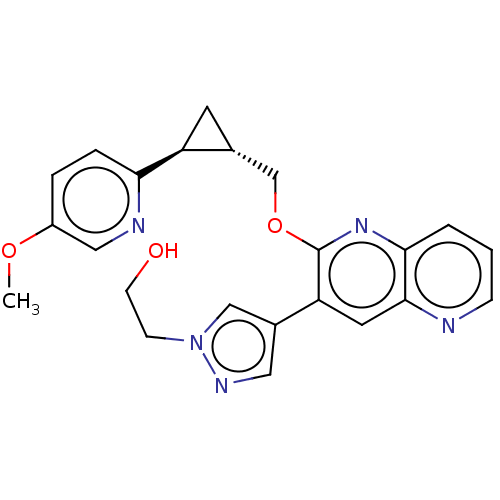
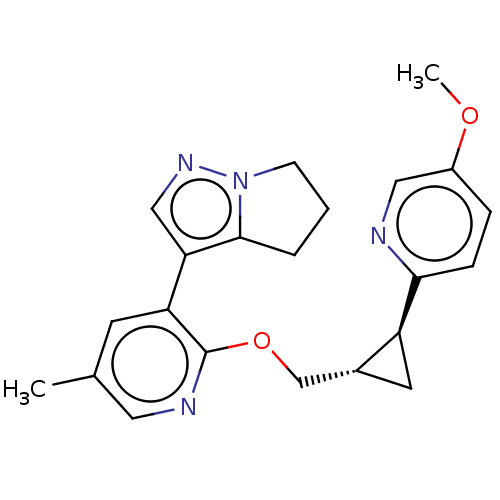
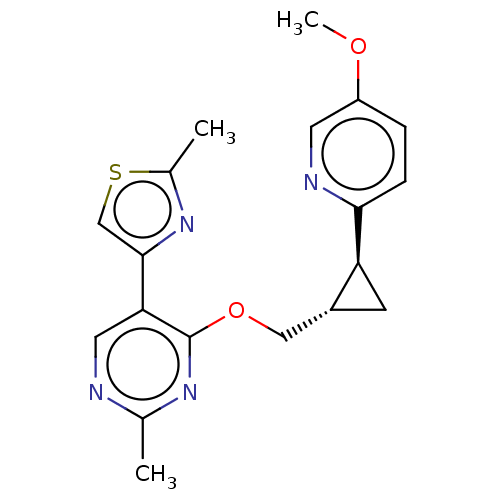
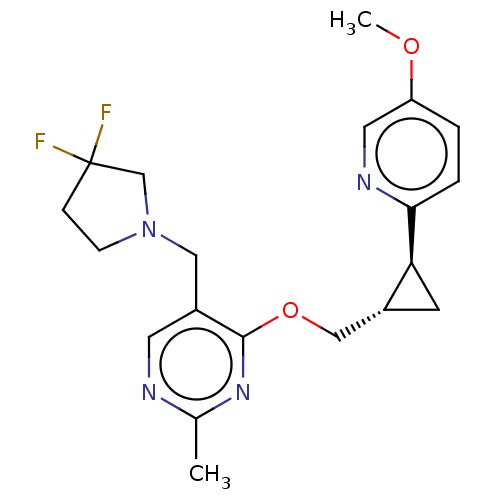
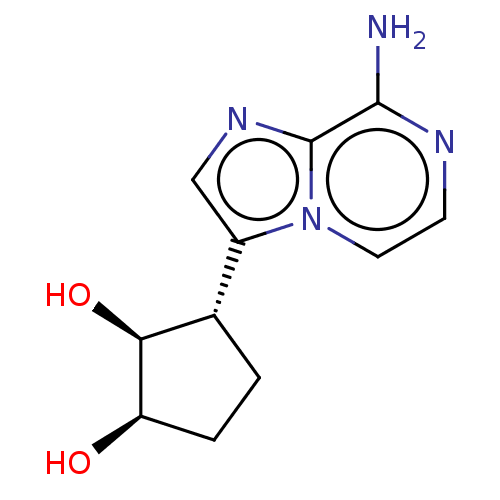
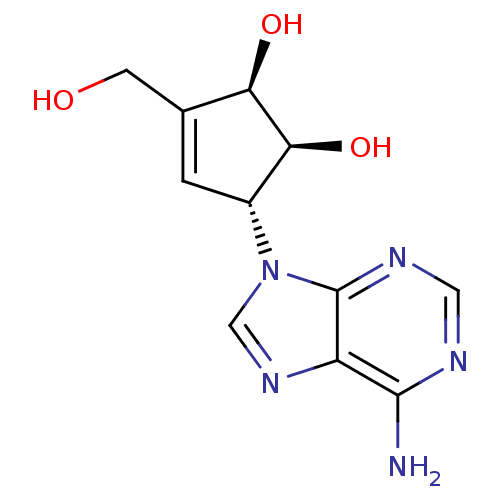
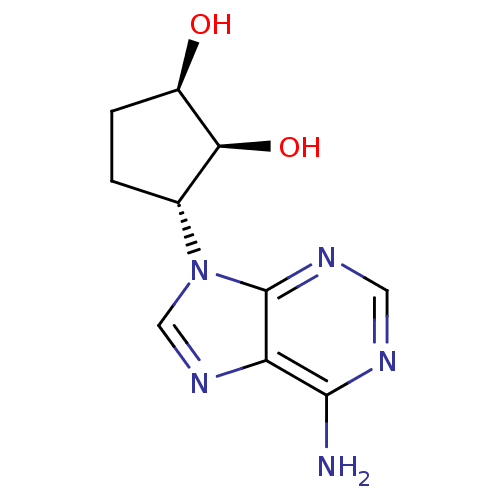
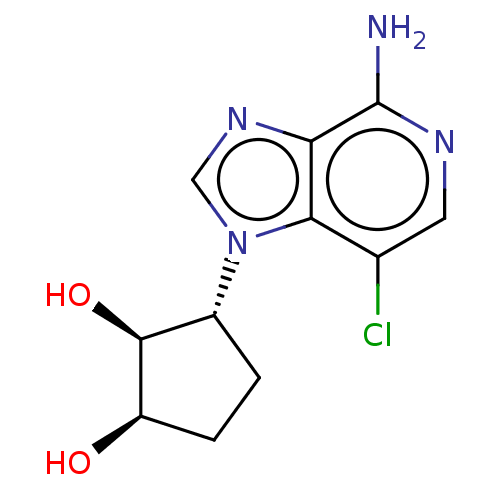
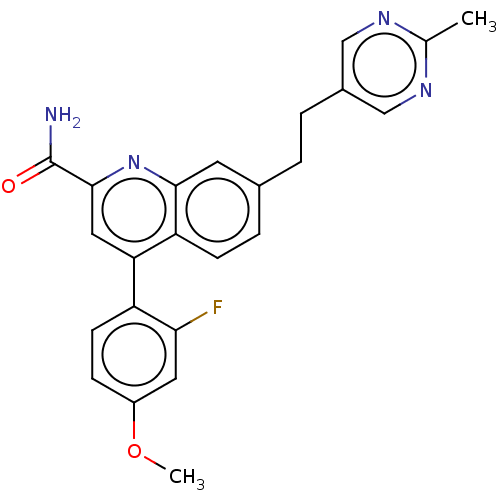
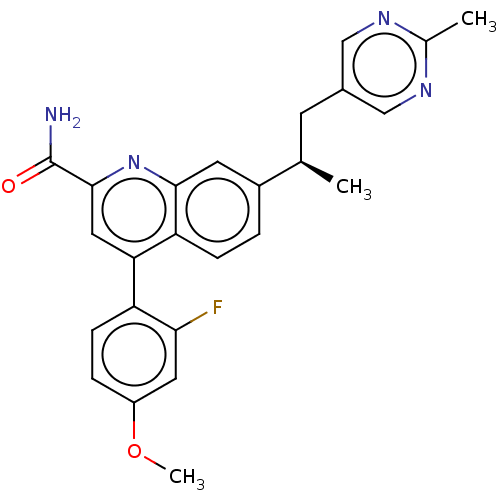
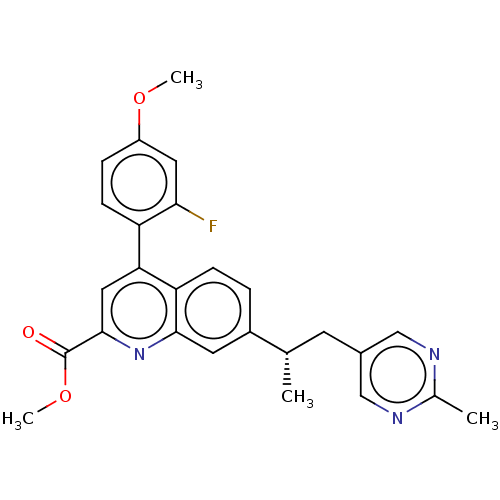
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.00200nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
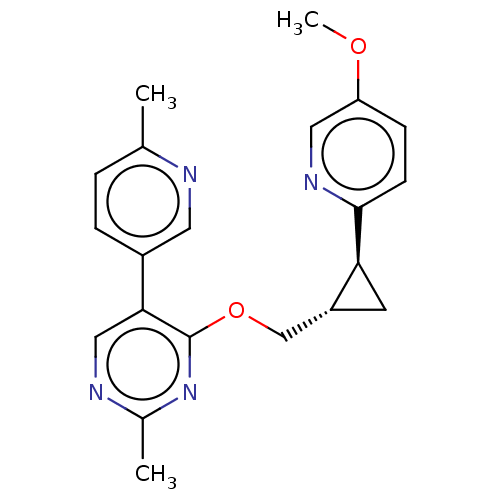
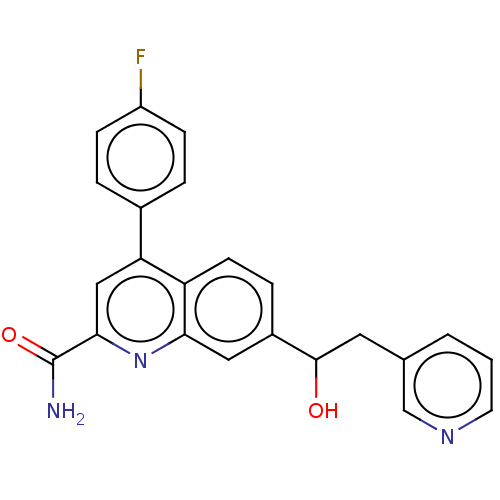
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.0400nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
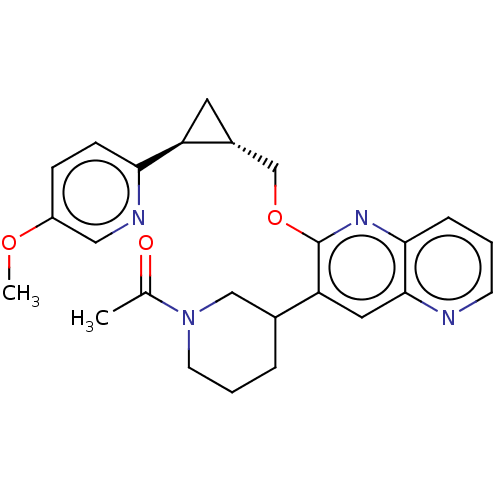
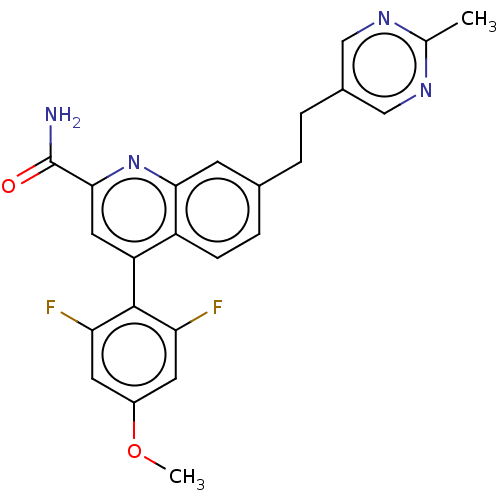
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.150nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
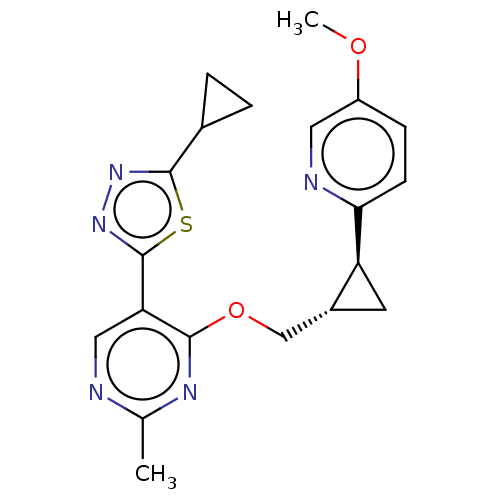
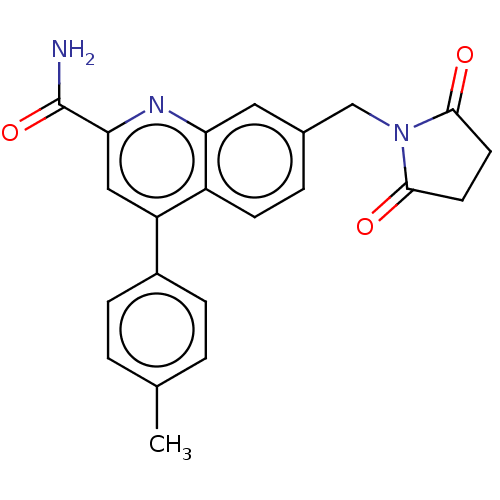
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.220nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.230nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.25nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.340nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.600nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.780nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 0.800nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 2.5nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 3.20nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 4.20nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 60.8nMAssay Description:In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Positive allosteric modulation of 5-HT2B receptor (unknown origin)More data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Positive allosteric modulation of platelet-activating factor receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair