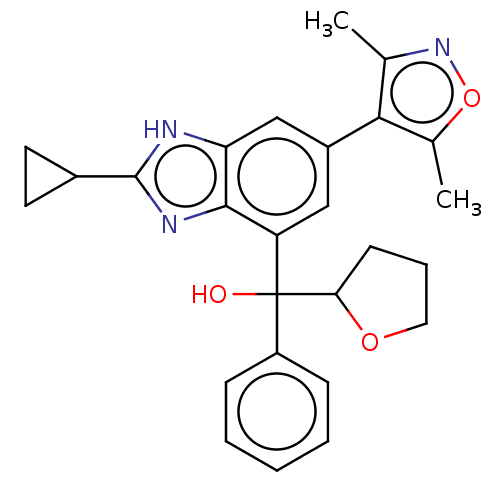
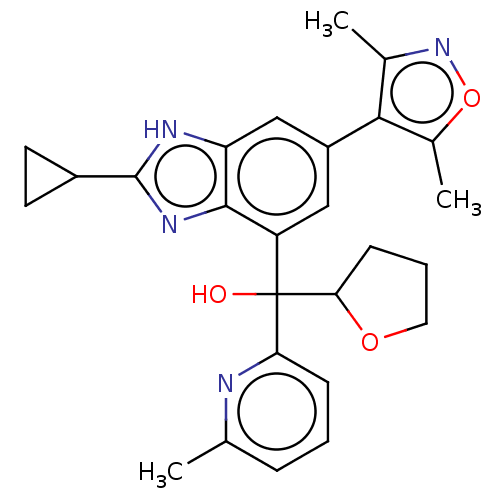
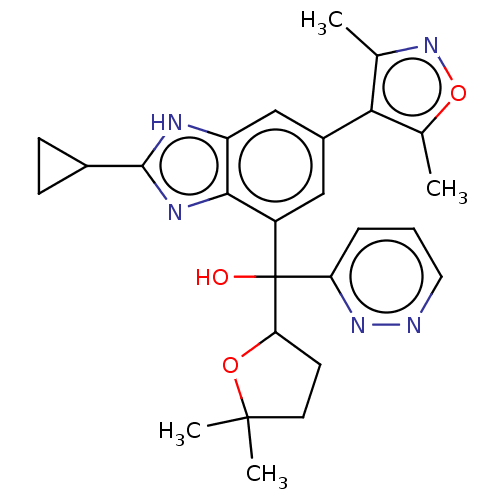
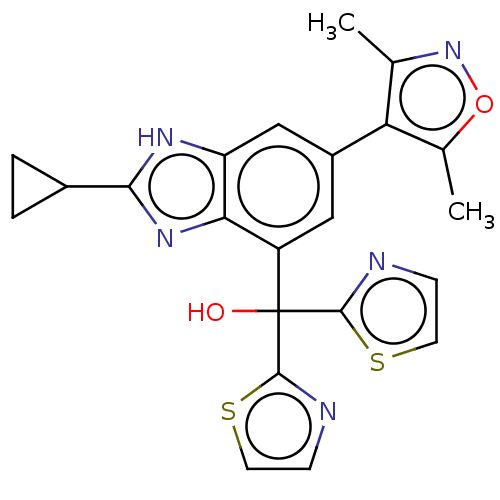
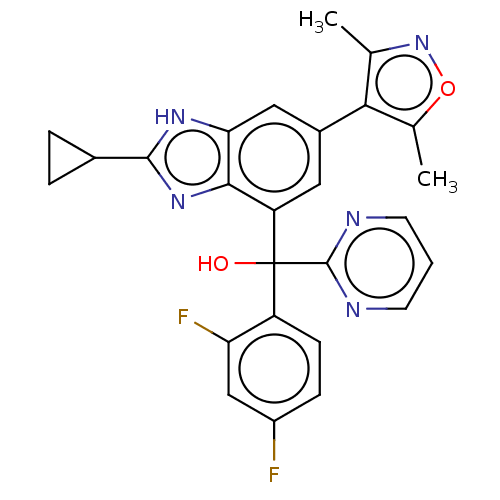
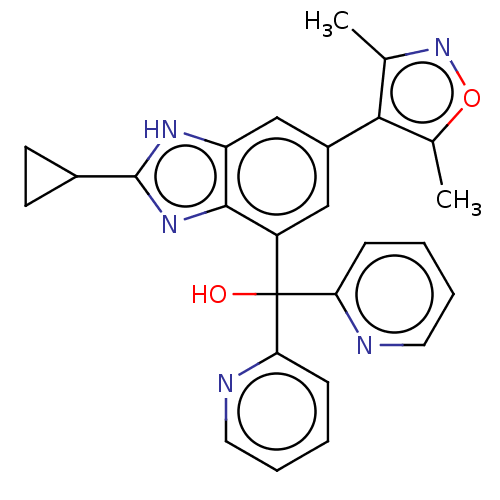
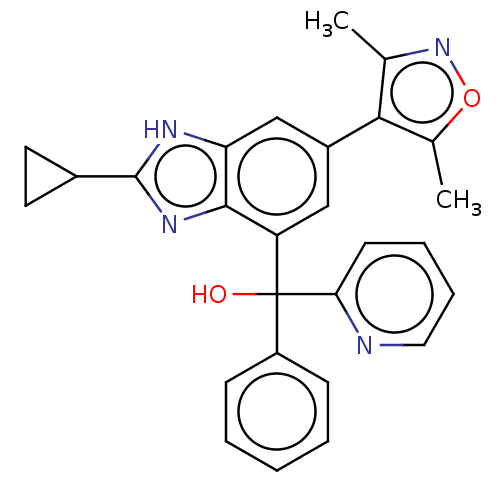
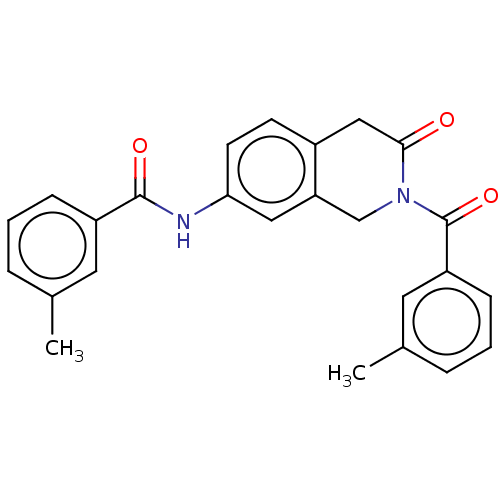
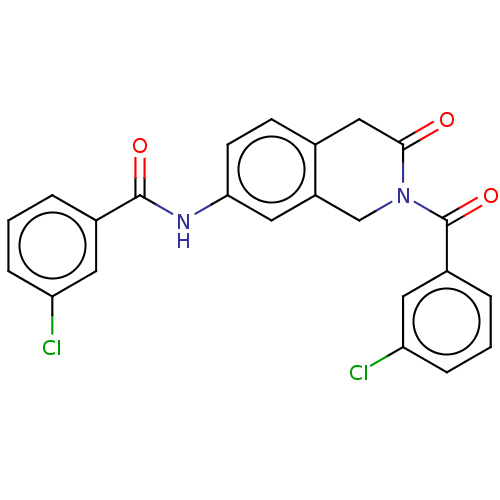
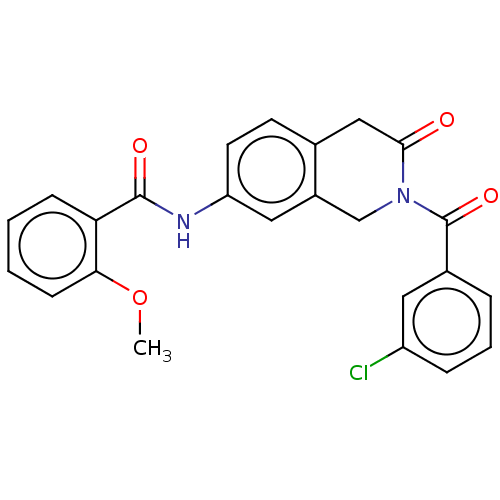
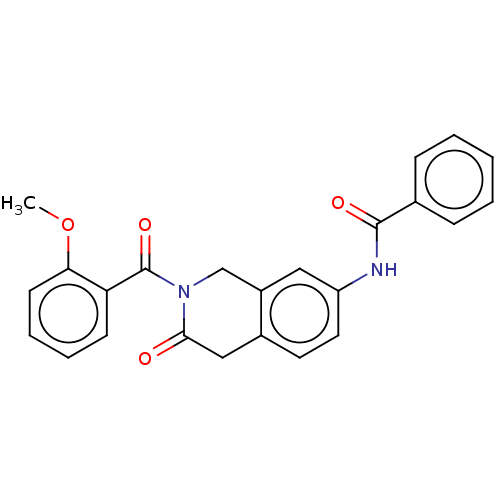
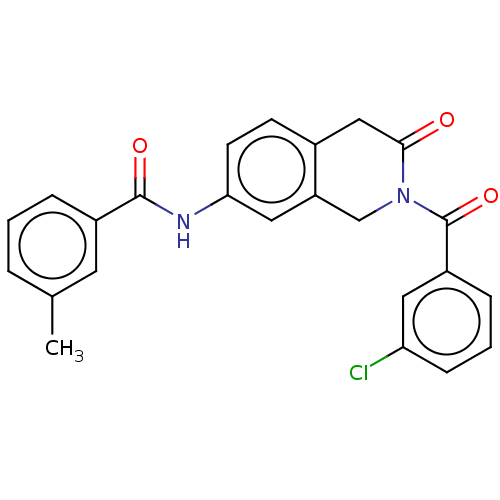
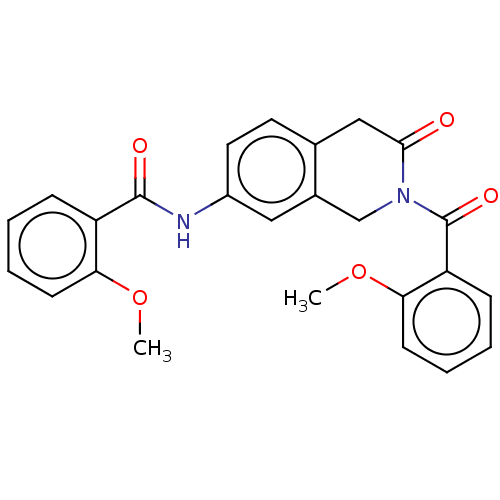
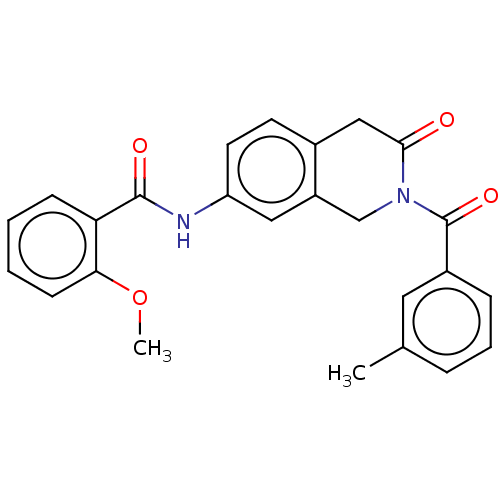
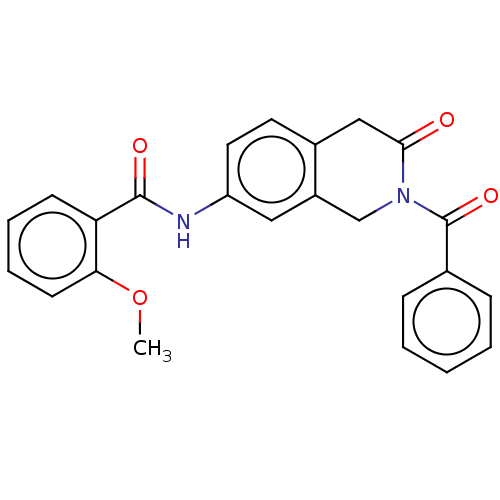
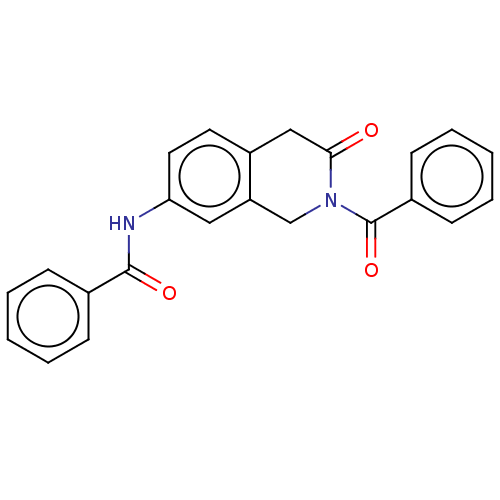
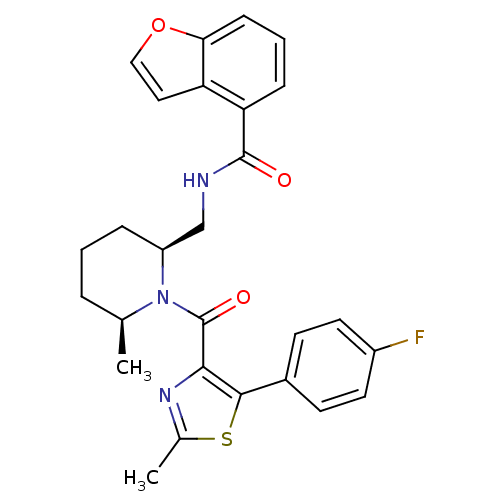
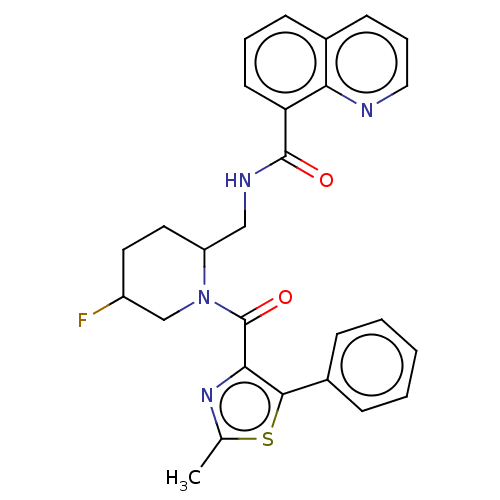
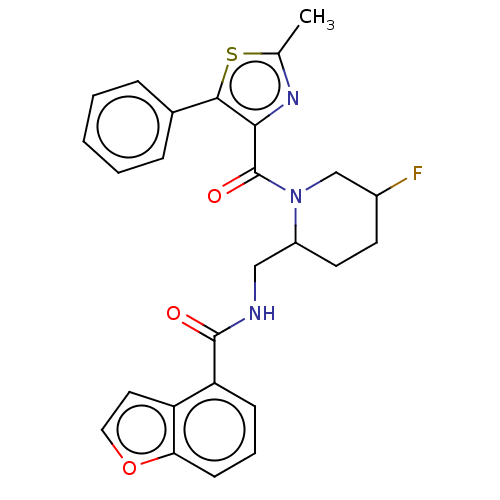
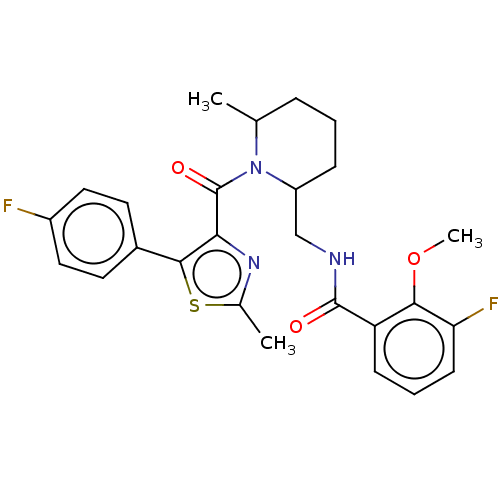
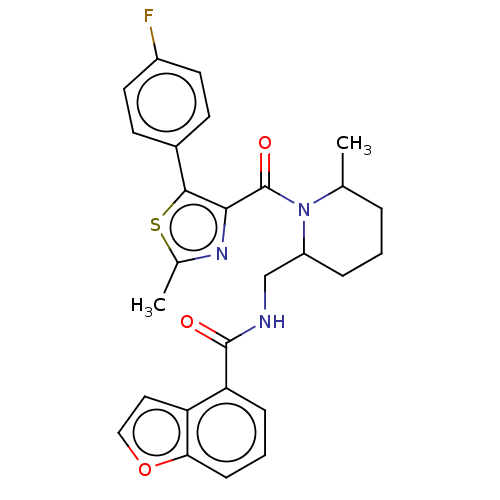
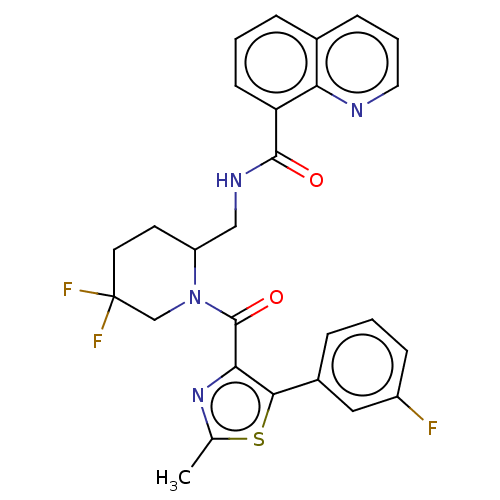
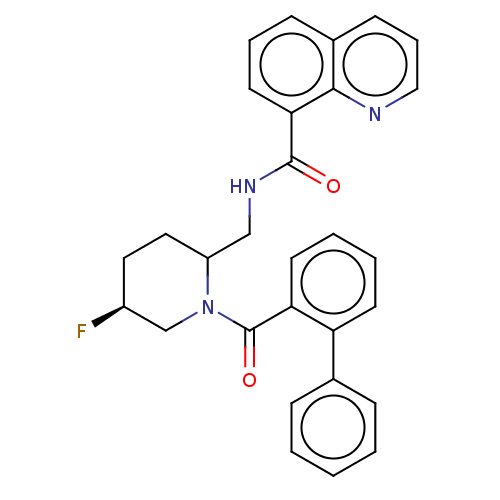
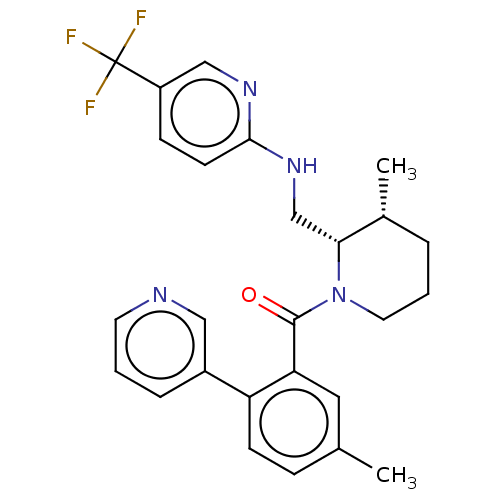
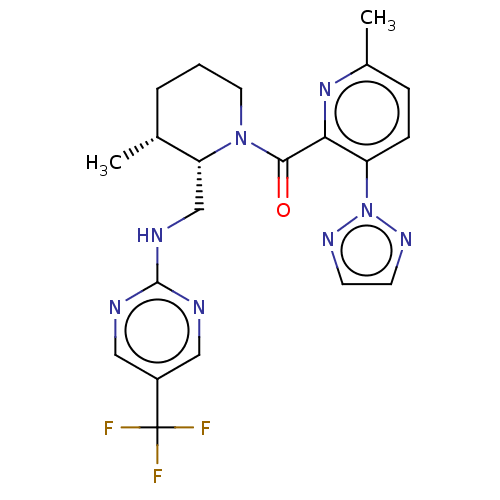
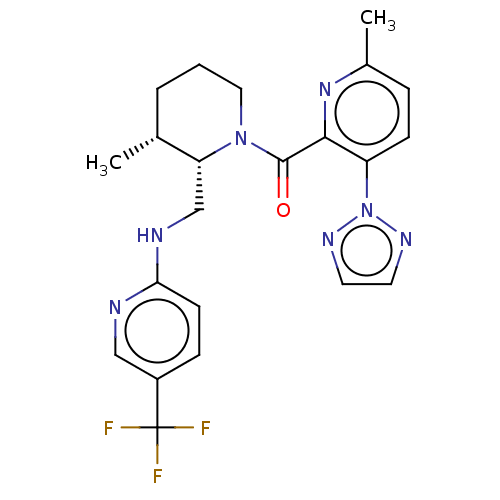
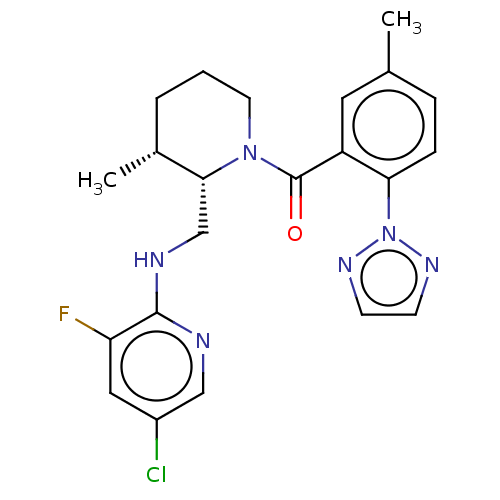
Affinity DataKi: 2nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 10.4nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 10.7nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 10.8nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 11.7nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 12.4nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 12.8nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 13.5nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataKi: 20.2nMAssay Description:Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0170nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0190nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0210nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0220nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0290nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00...More data for this Ligand-Target Pair