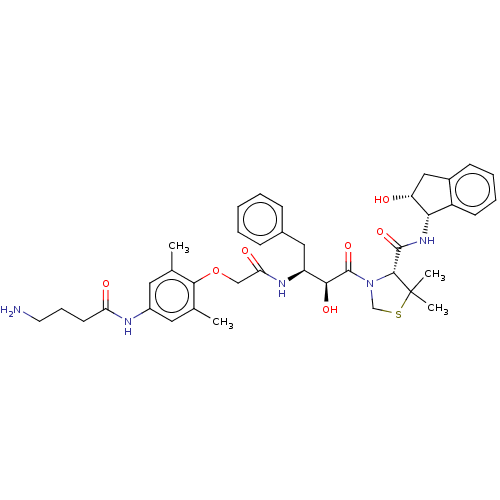
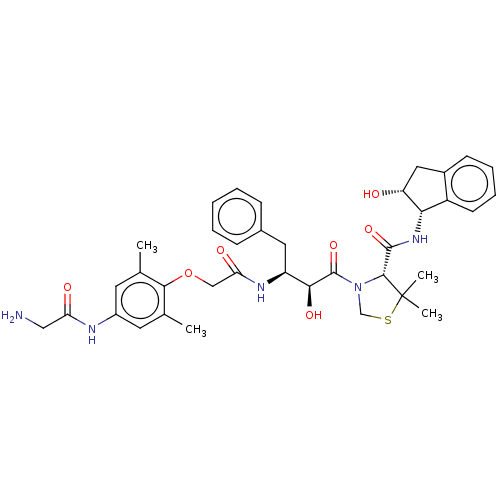
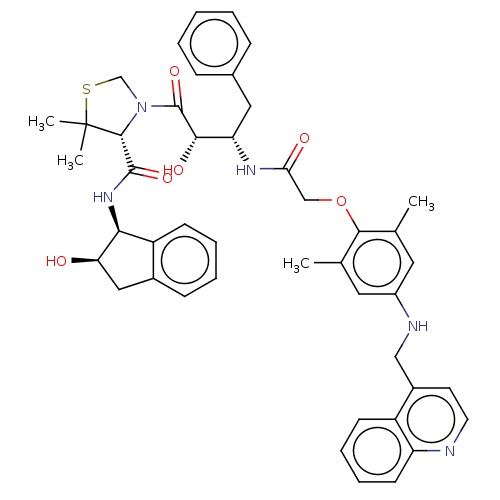
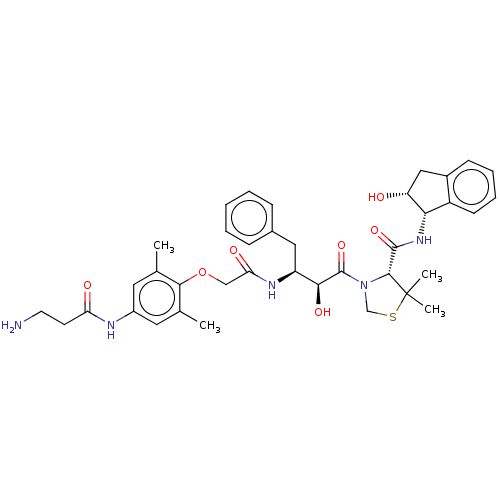
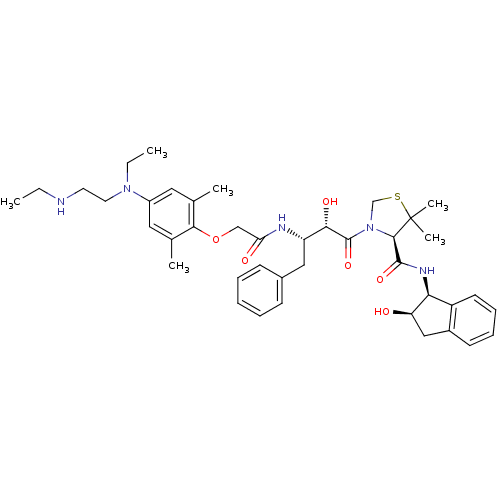
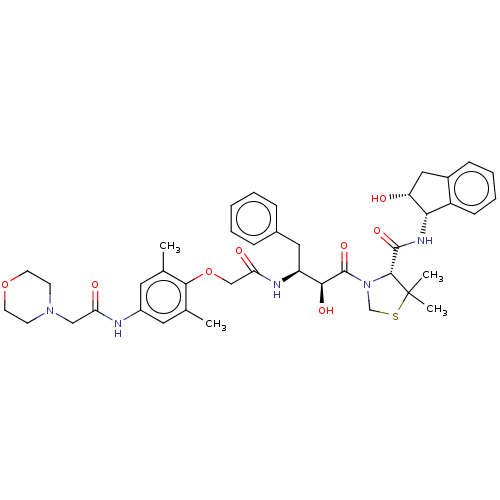
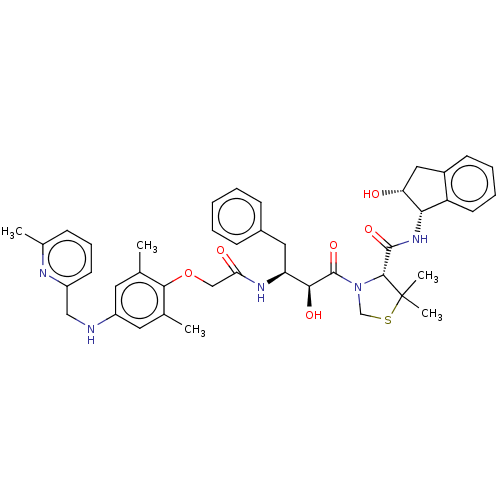
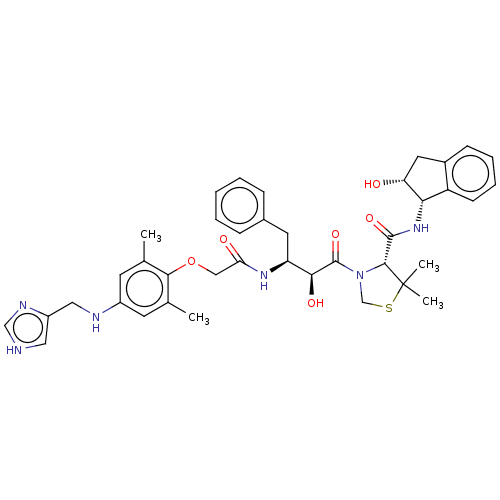
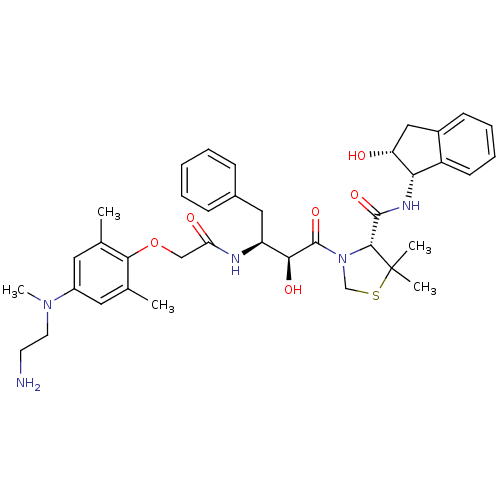
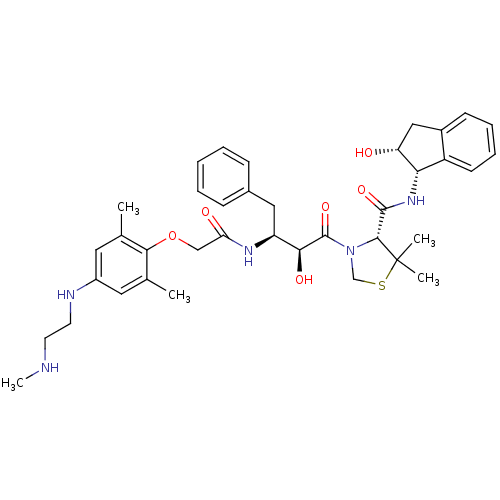
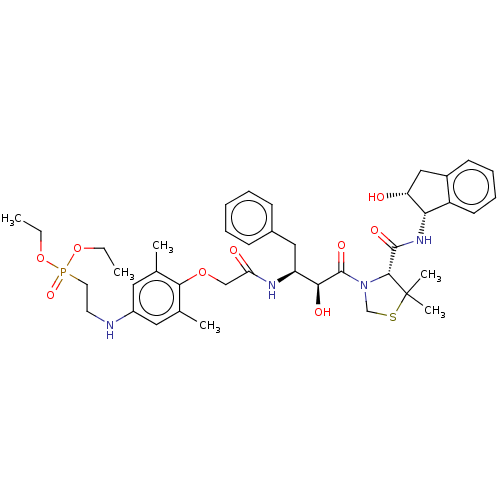
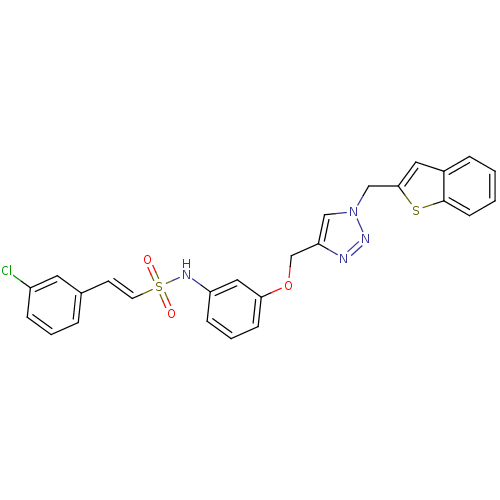
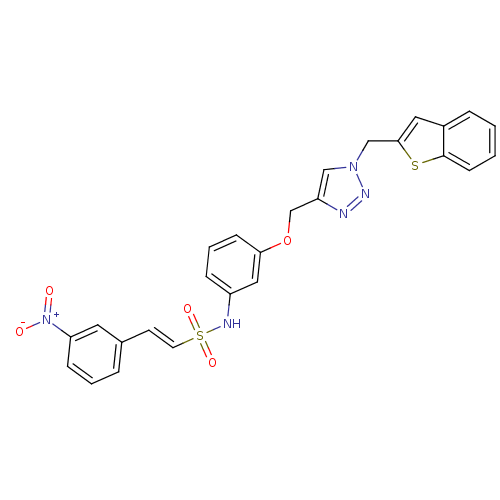
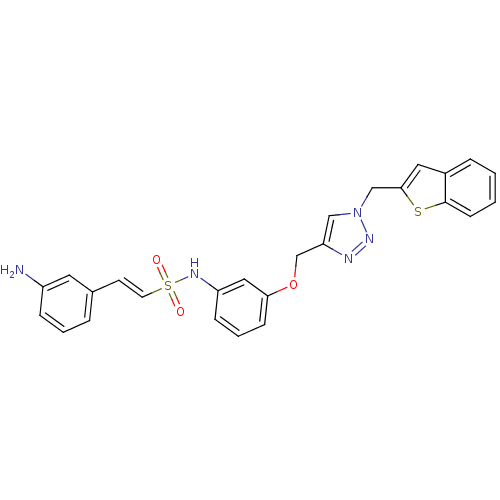
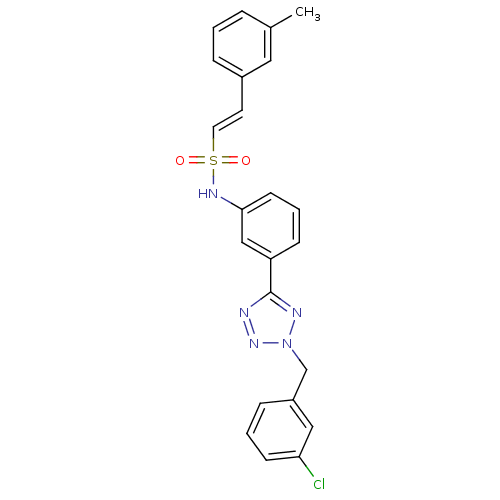
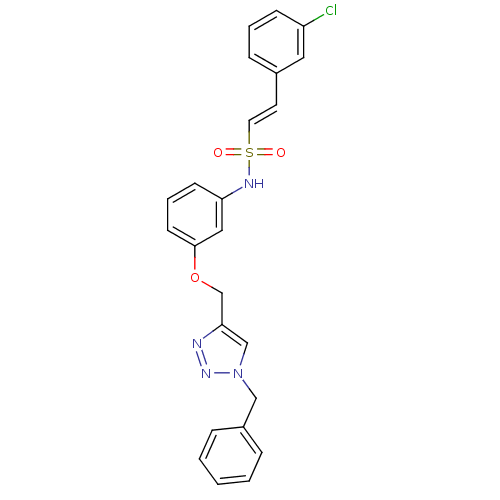
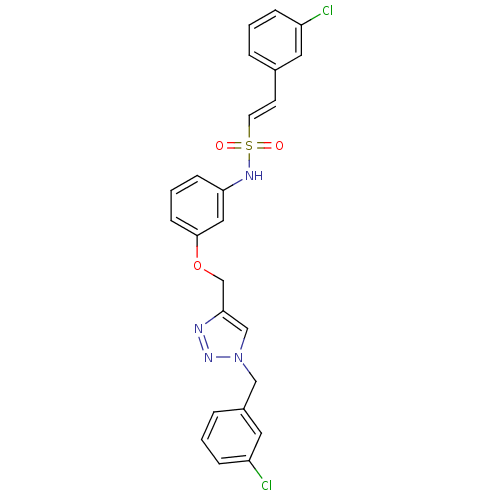
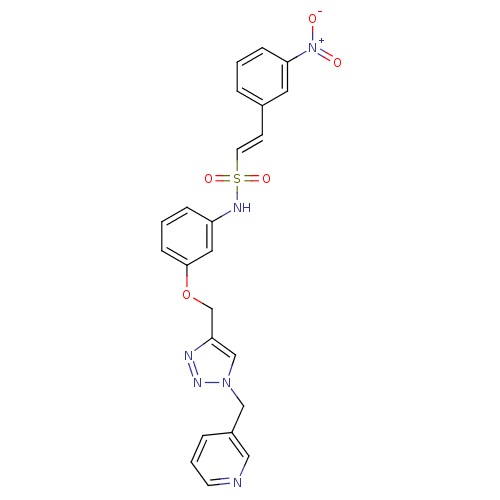
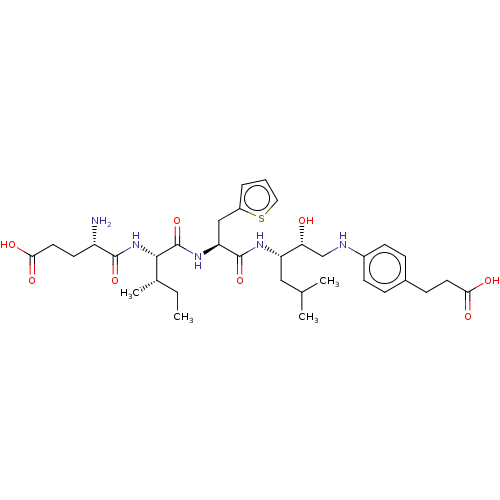
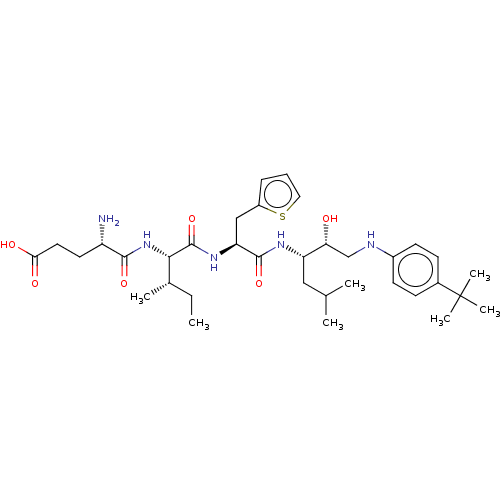
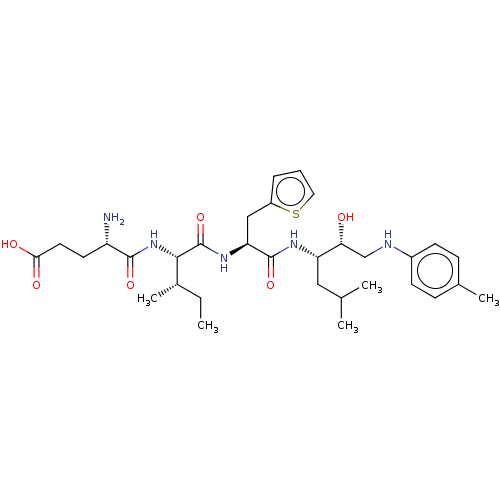
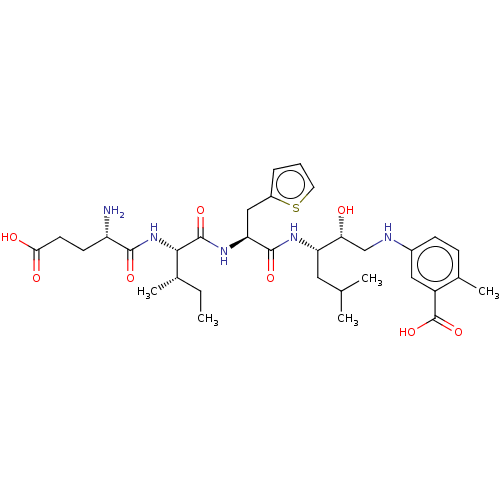
Affinity DataKi: 0.100nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 8.5nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 9.10nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 880nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.71E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.75E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.97E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 4.60E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 5.43E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 6.89E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 8.91E+3nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.37E+4nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 3.92E+4nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 6.25E+4nMAssay Description:Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Binding affinity to human recombinant BACE-1 (1 to 460 residue) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition...More data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibition of recombinant BACE-1 (unknown origin) using H-Ile-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-NH2 peptide...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)