Affinity DataKi: 0.00110nM IC50: 1.00E+3nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00182nM IC50: 1.21E+4nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00216nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00256nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00425nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00597nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00838nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.0118nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
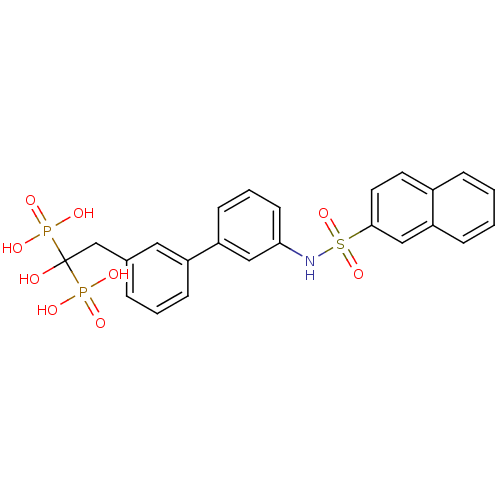
Affinity DataKi: 0.430nMAssay Description:Binding affinity against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
National Yang-Ming University
National Yang-Ming University
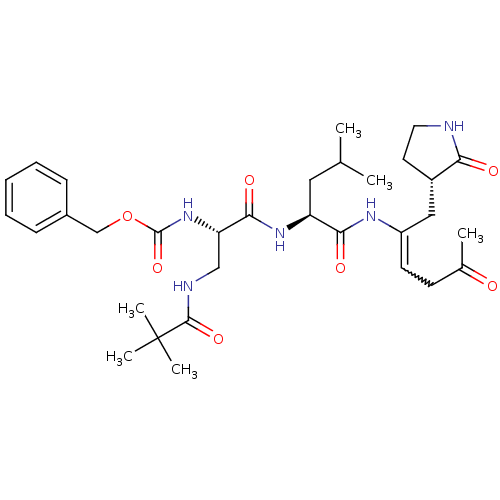
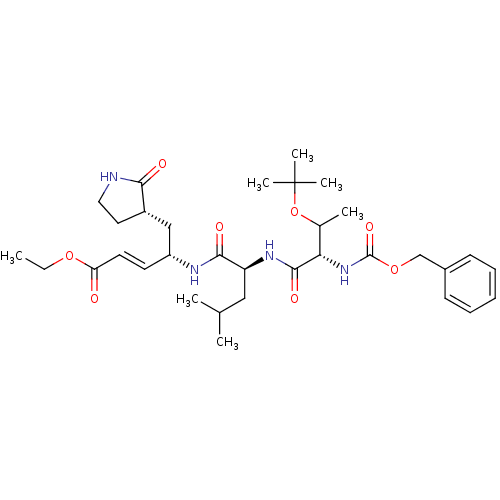
Affinity DataKi: 38nM ΔG°: -42.4kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
National Yang-Ming University
National Yang-Ming University
Affinity DataKi: 54nM ΔG°: -41.5kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
National Yang-Ming University
National Yang-Ming University
Affinity DataKi: 58nM ΔG°: -41.3kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 83nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
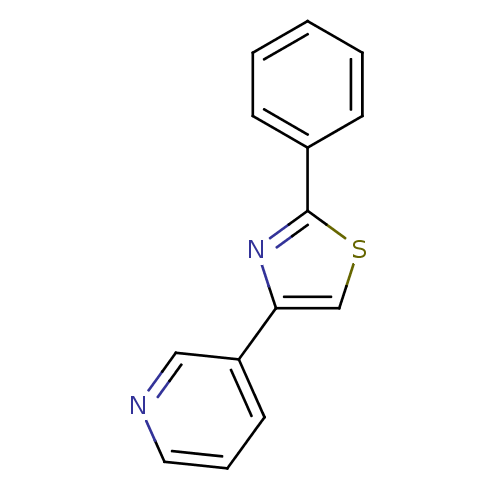
Affinity DataKi: 90nMAssay Description:Inhibitory activity against L-arginine binding to Inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
National Yang-Ming University
National Yang-Ming University
Affinity DataKi: 99nM ΔG°: -40.0kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 234nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 285nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 400nM ΔG°: -36.5kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
Affinity DataKi: 800nM ΔG°: -34.8kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
Affinity DataKi: 1.16E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.48E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nM ΔG°: -33.2kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
Affinity DataKi: 1.72E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nM ΔG°: -32.0kJ/molepH: 6.5 T: 2°CAssay Description:Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a...More data for this Ligand-Target Pair
Affinity DataKi: 2.55E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair

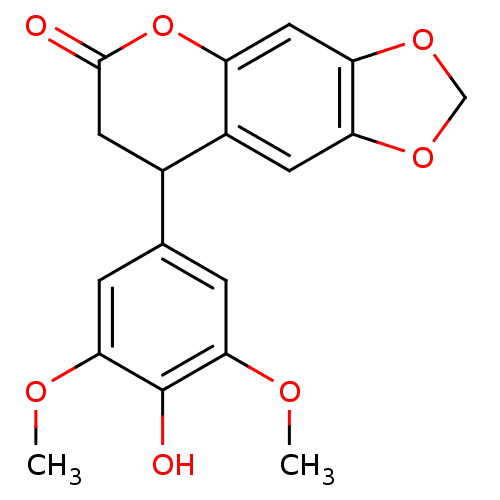
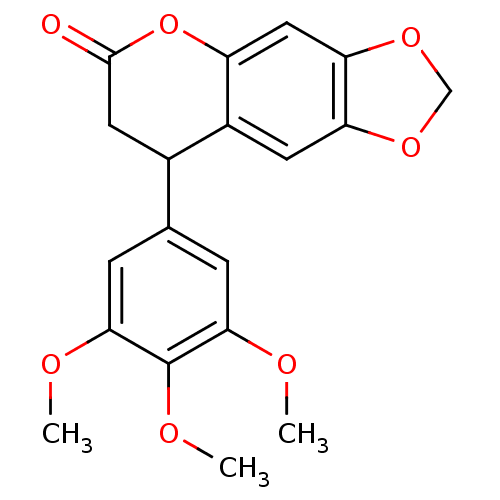
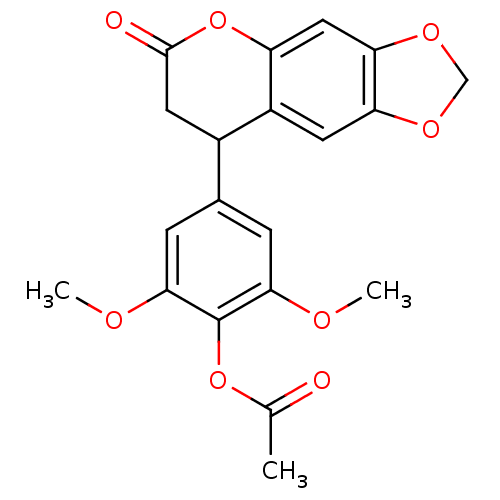
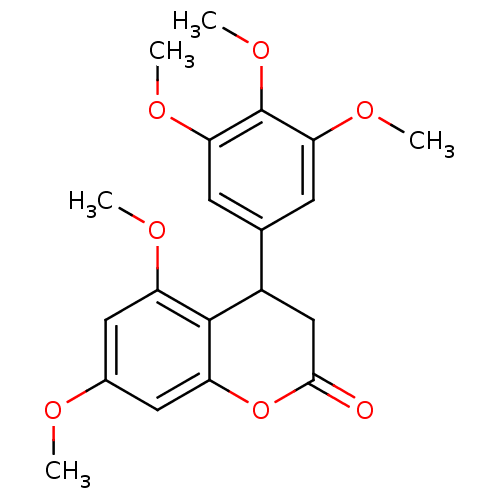
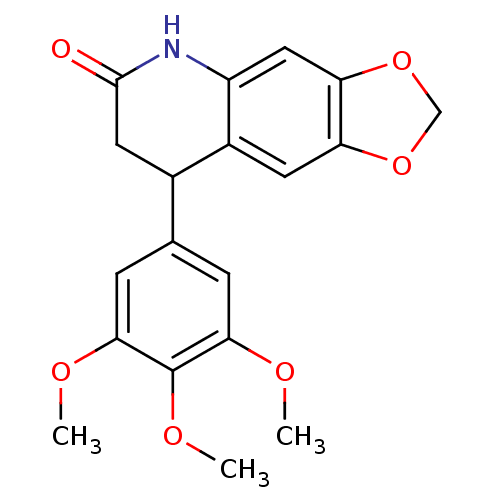
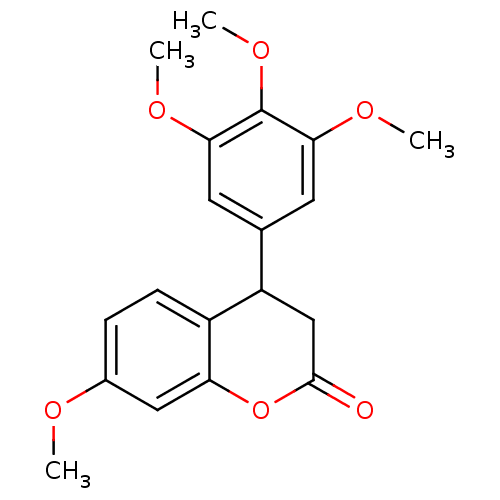
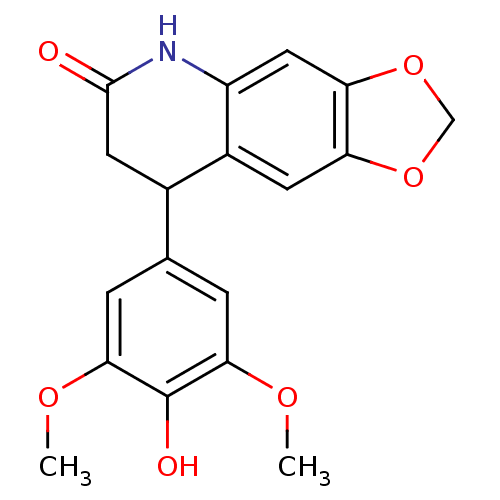
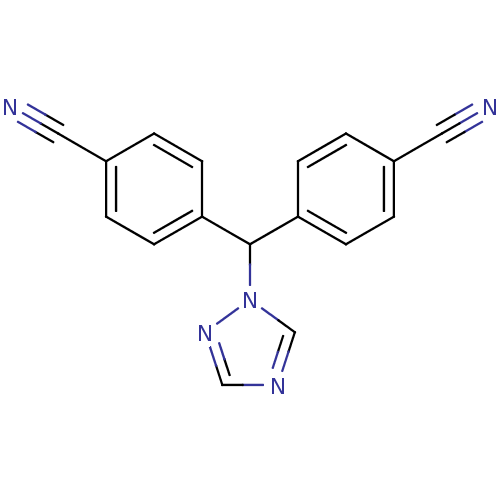





 3D Structure (crystal)
3D Structure (crystal)