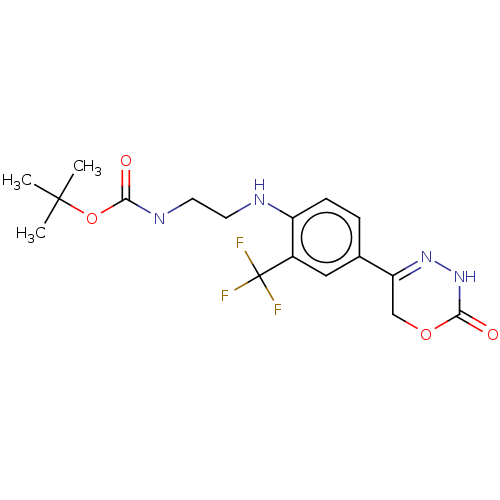
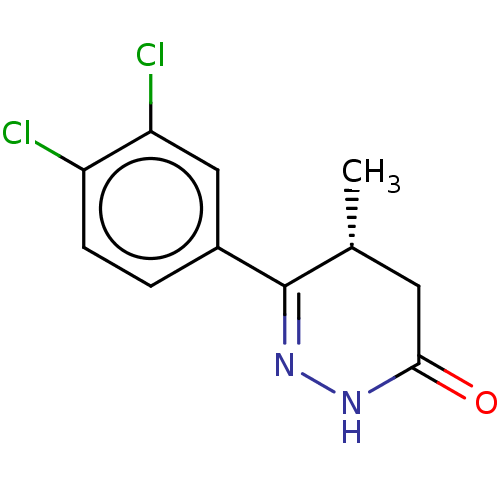
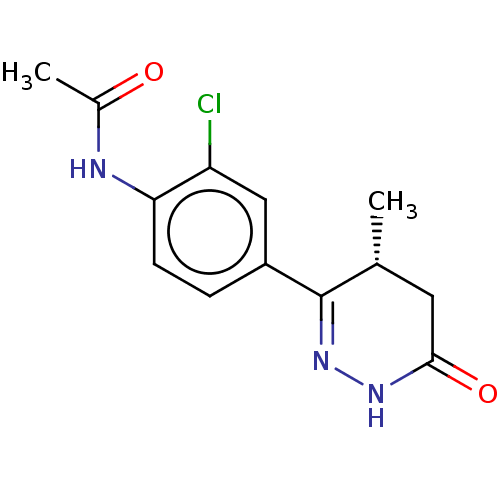
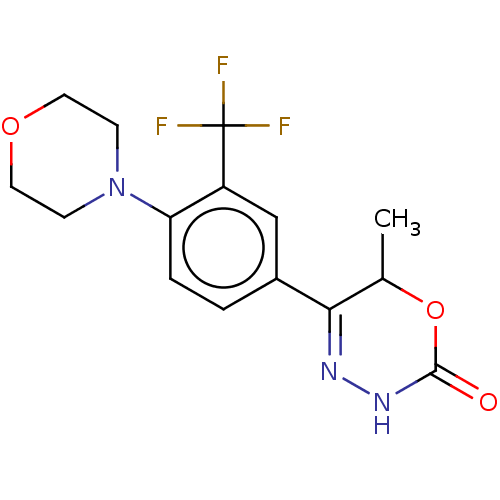
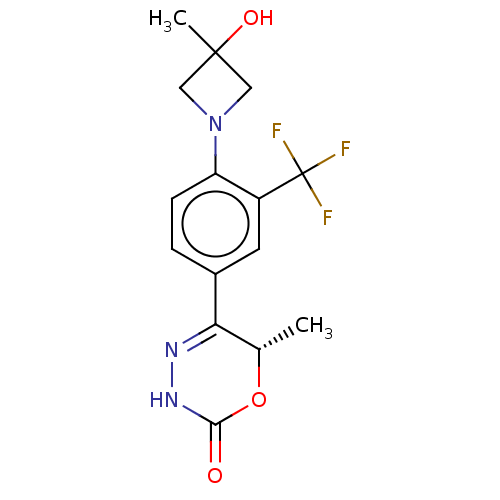
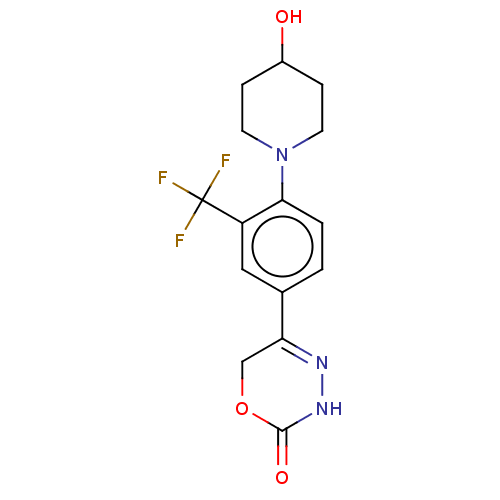
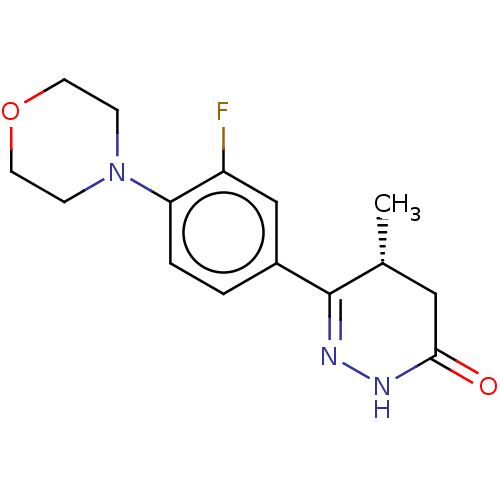
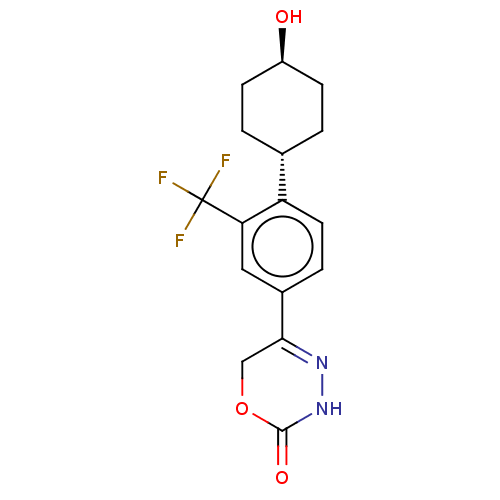
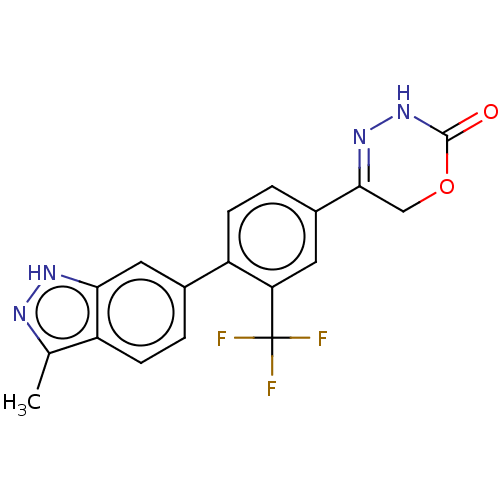
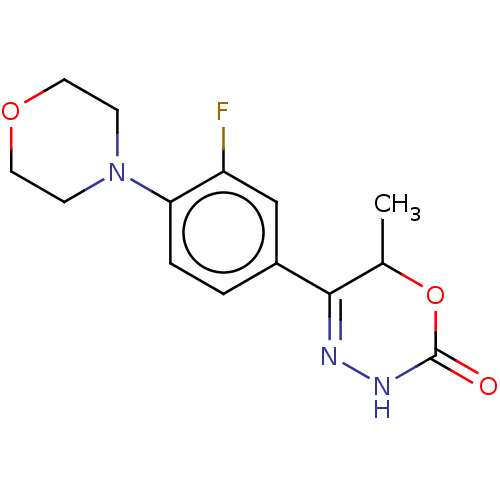
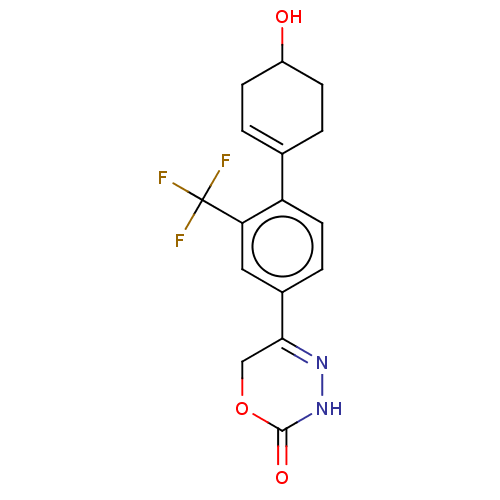
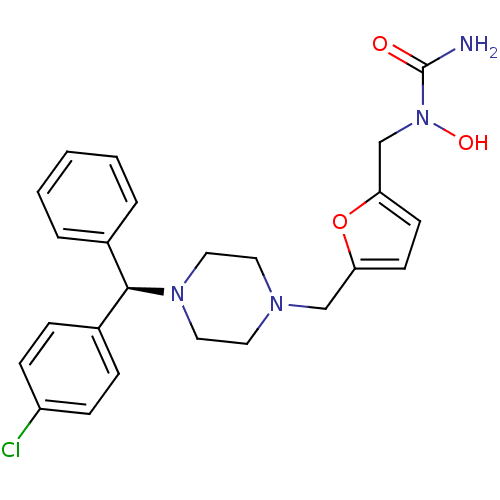
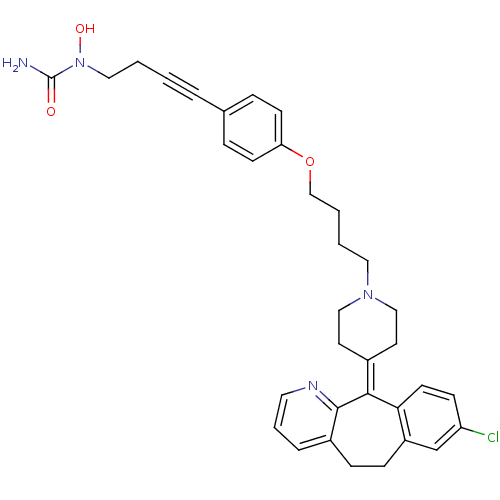
Affinity DataKi: 0.970nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
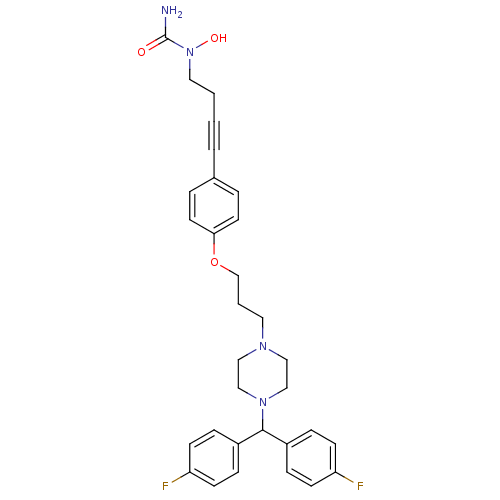
Affinity DataKi: 3.63nMAssay Description:Binding affinity for recombinant human Histamine H1 receptor expressed in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.01nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.89nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.89nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7.59nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.55nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 17.8nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 109nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 295nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 305nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 414nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 550nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 660nMAssay Description:In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
University Of Tennessee Health Science Center
Curated by ChEMBL
University Of Tennessee Health Science Center
Curated by ChEMBL
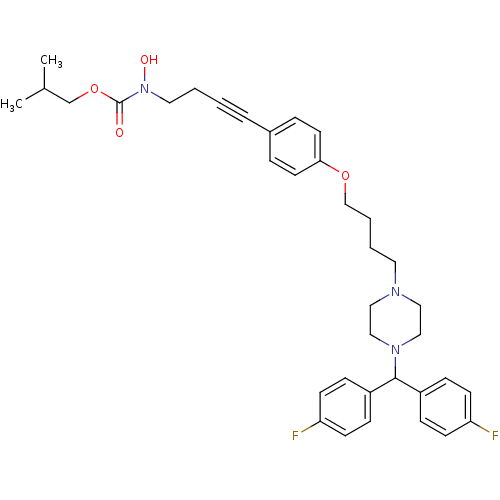
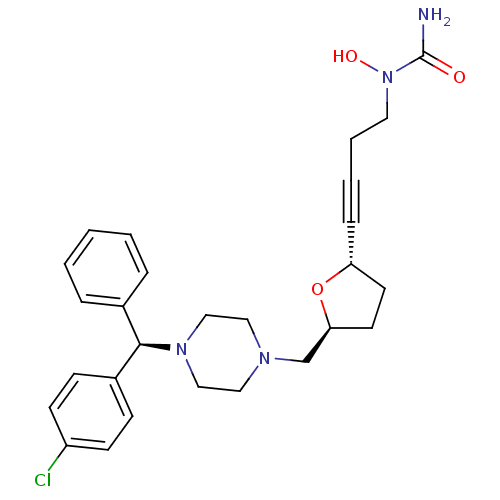
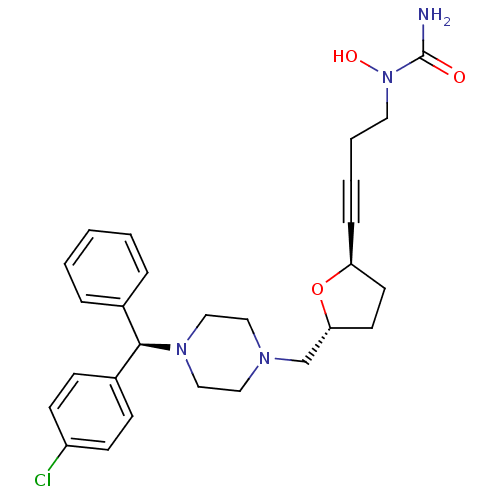
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human ENT1 expressed in porcine PK15NTD cells by cell-based [3H]5-uridine uptake assayMore data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
University Of Tennessee Health Science Center
Curated by ChEMBL
University Of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human ENT1 expressed in porcine PK15NTD cells by cell-based [3H]5-uridine uptake assayMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
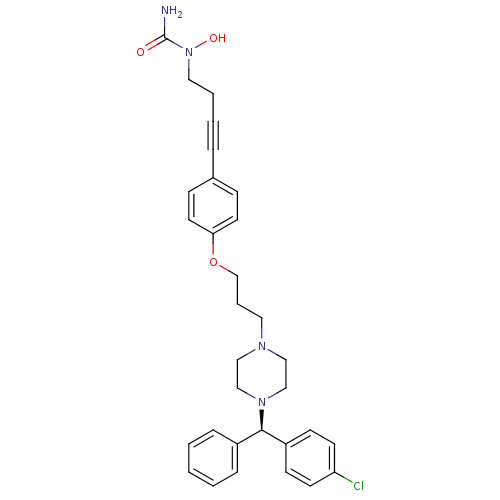
Affinity DataIC50: 4nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
Broad Institute
Curated by ChEMBL
Broad Institute
Curated by ChEMBL
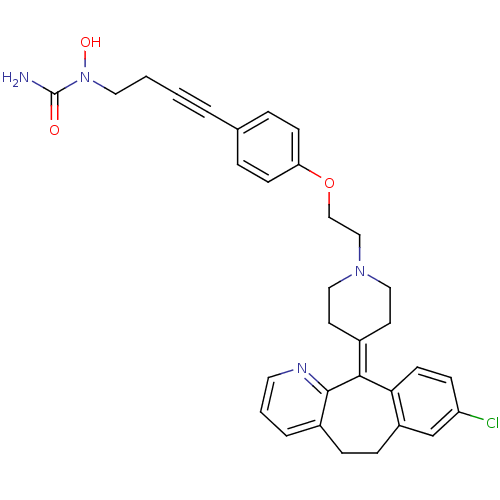
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant DHODHMore data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
University Of Tennessee Health Science Center
Curated by ChEMBL
University Of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human ENT1 expressed in porcine PK15NTD cells by cell-based [3H]5-uridine uptake assayMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 6nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 6nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 7nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:Inhibition of human N-terminal His-GST-tagged recombinant PDE3A (669-end residues) using fluorescent labelled cAMP as substrateMore data for this Ligand-Target Pair
Ligand Info
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:Inhibition of human N-terminal His-GST-tagged recombinant PDE3A (669-end residues) using fluorescent labelled cAMP as substrateMore data for this Ligand-Target Pair
Ligand Info
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant PDE3BMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:Inhibition of human N-terminal His-GST-tagged recombinant PDE3A (669-end residues) using fluorescent labelled cAMP as substrateMore data for this Ligand-Target Pair
Ligand Info
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
Broad Institute
Curated by ChEMBL
Broad Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant DHODH expressed in baculovirus infected insect cells using dihydroorotate as substrate in presence of quinone by dich...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 12nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 13nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 13nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 13nMAssay Description:The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determina...More data for this Ligand-Target Pair










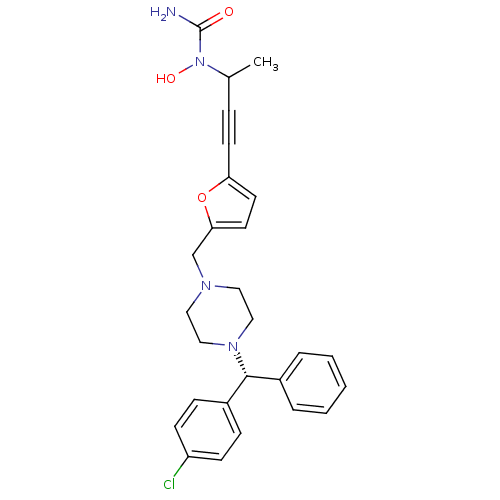
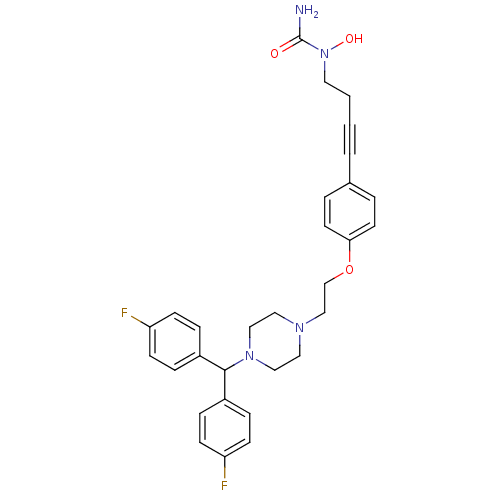
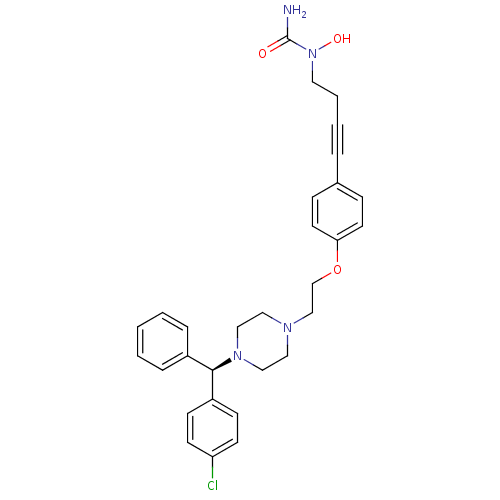
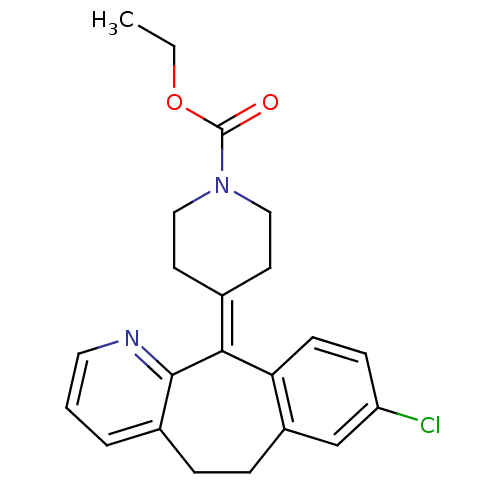
 3D Structure (crystal)
3D Structure (crystal)