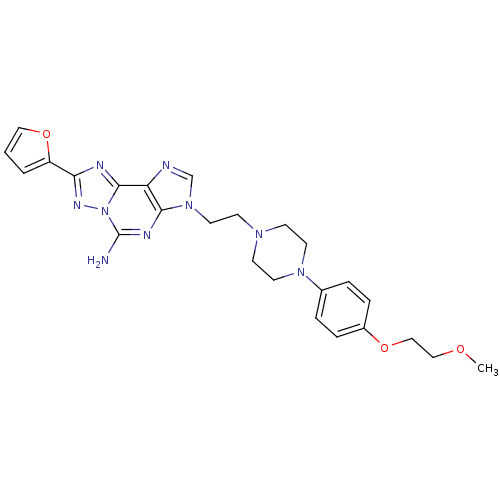
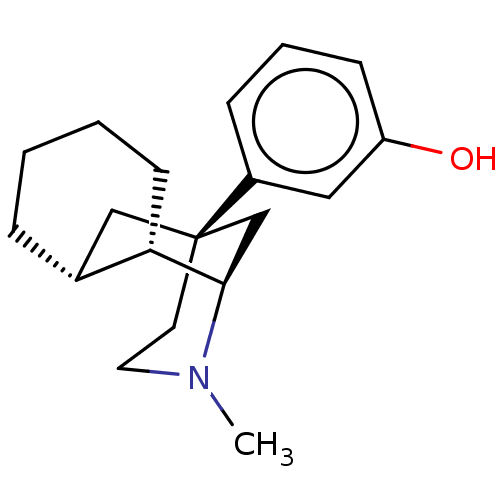
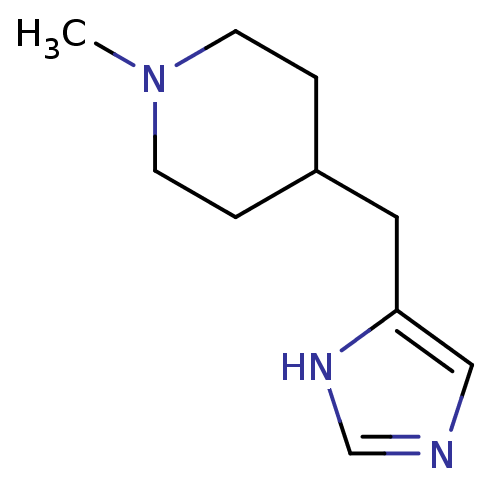
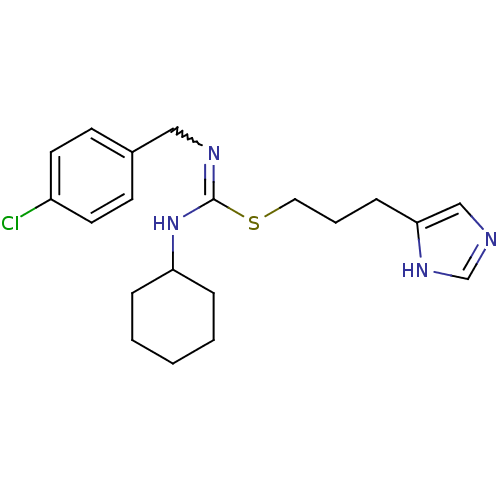
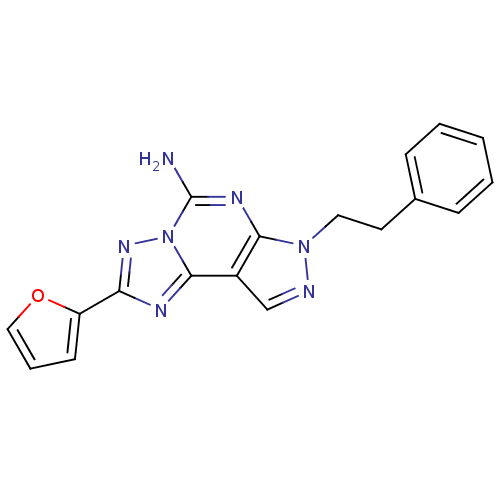
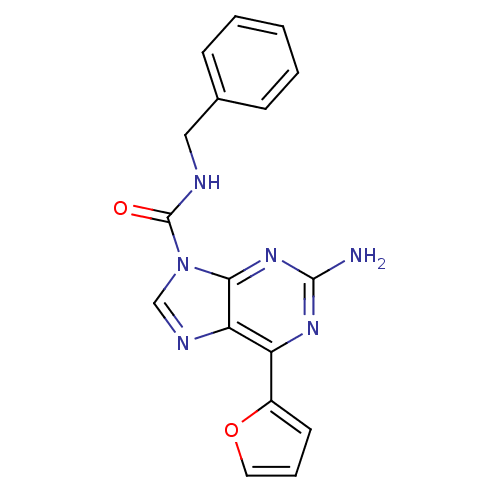
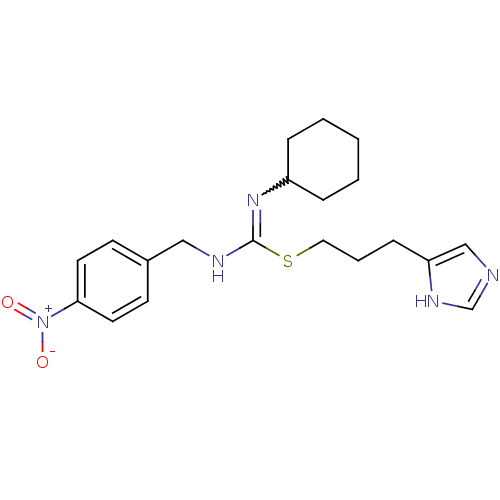
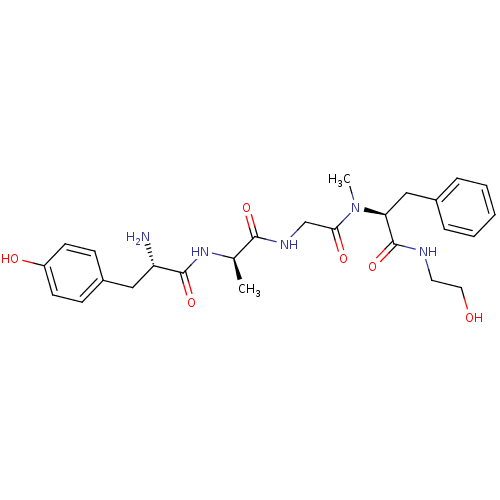
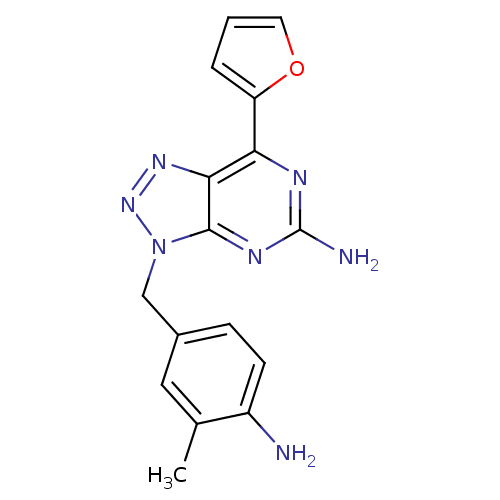
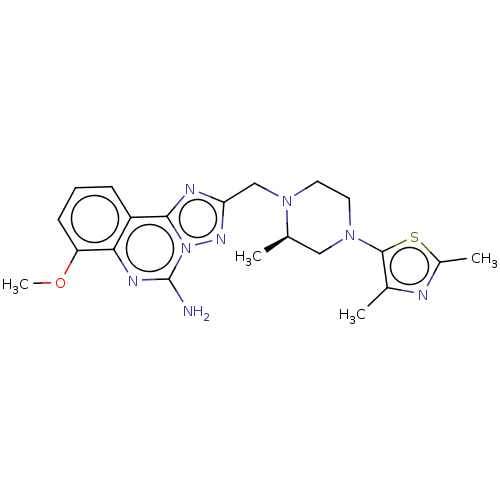
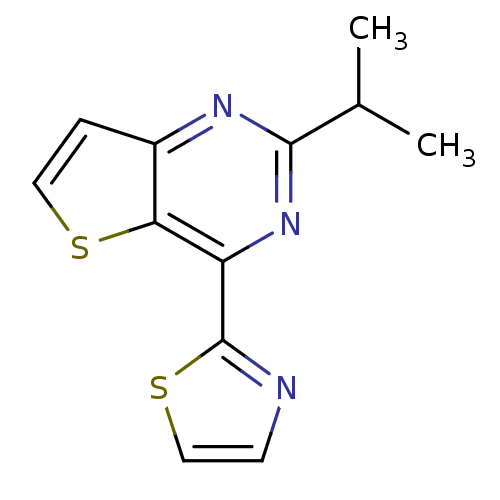
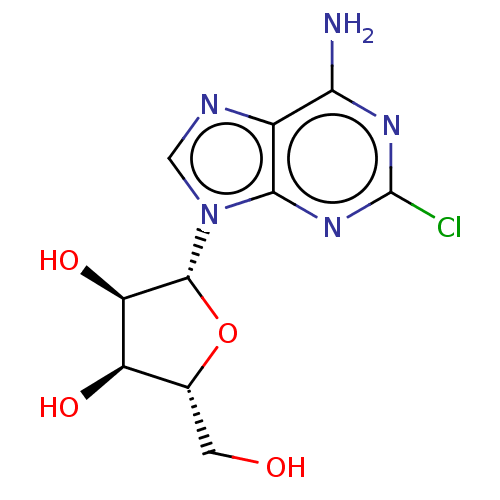
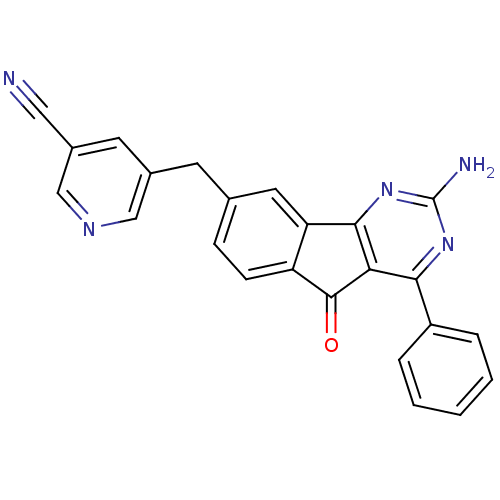
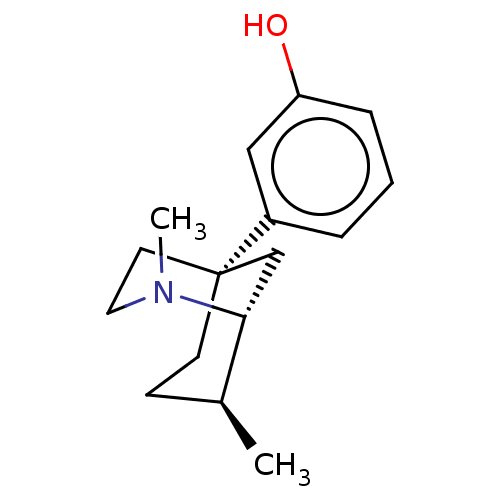
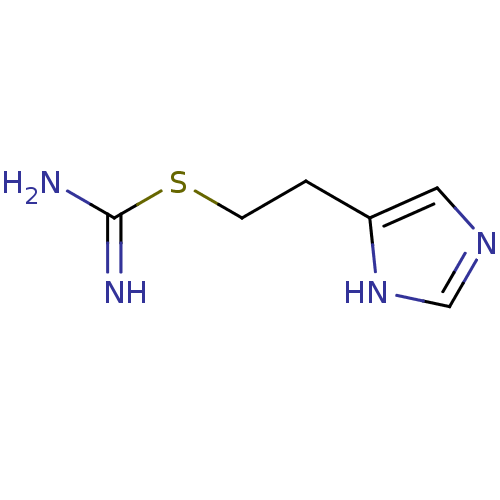
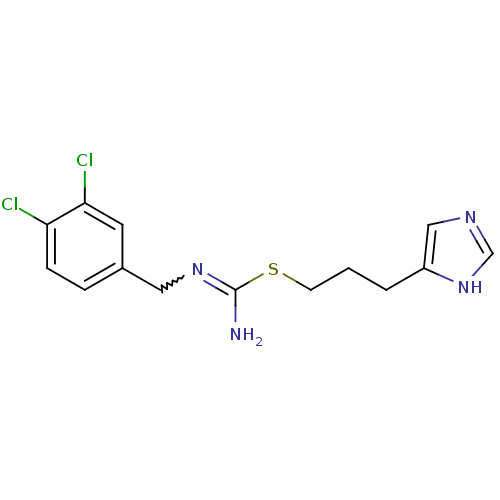
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human recombinant adenosine receptor A2a by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Binding affinity for rat Adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of rat recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Antagonist activity at human recombinant adenosine A1 receptor by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 0.501nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.501nMAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 0.794nMAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataKi: 1.02nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.05nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 1.15nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Binding affinity to human recombinant adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of MMP13-mediated collagen degradation by SDS-PAGEMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
National Institute On Drug Abuse And The National Institute On Alcohol Abuse And Alcoholism
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Agonistic activity at histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataKi: 1.62nMAssay Description:Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair


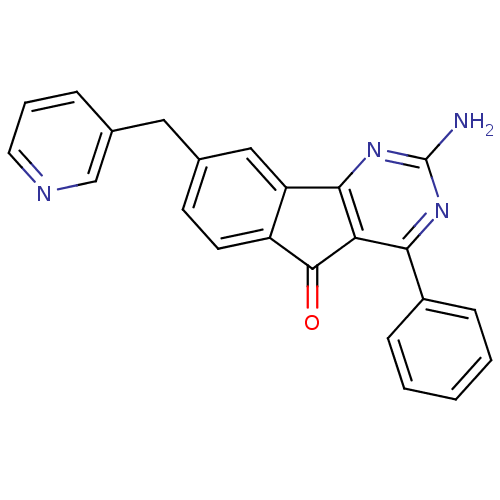
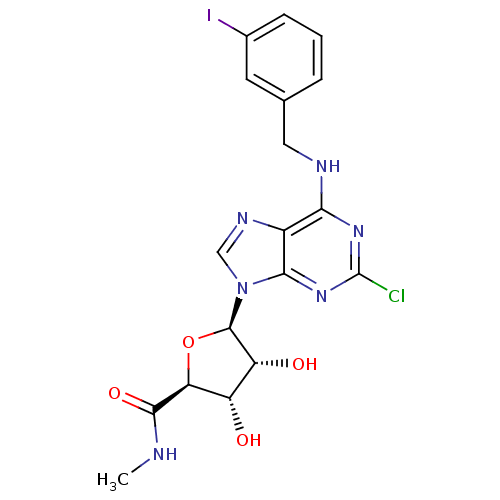
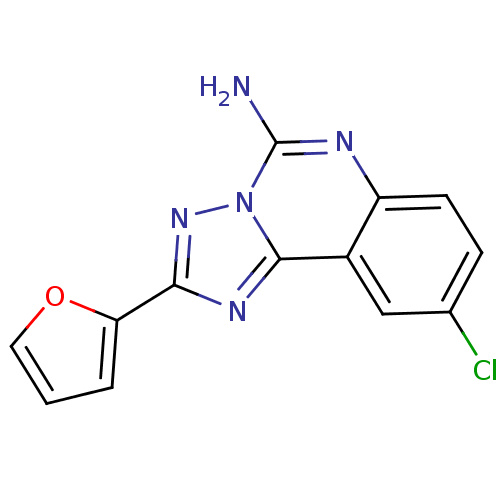
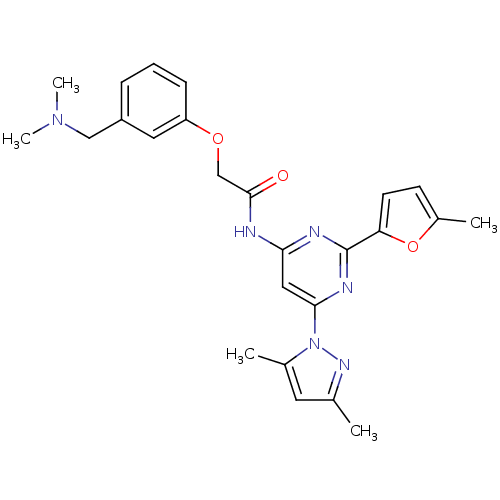
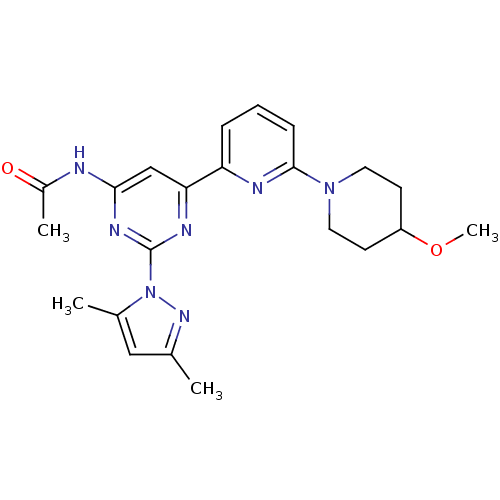

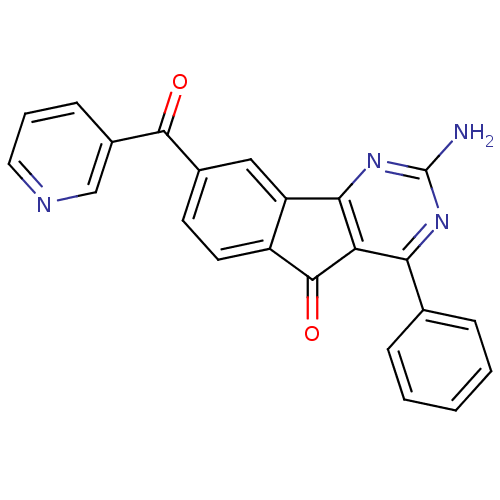
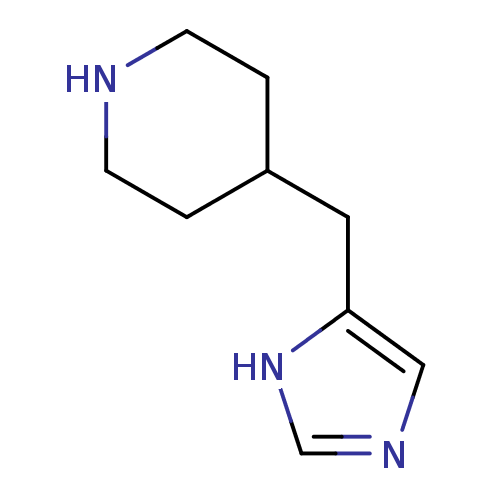
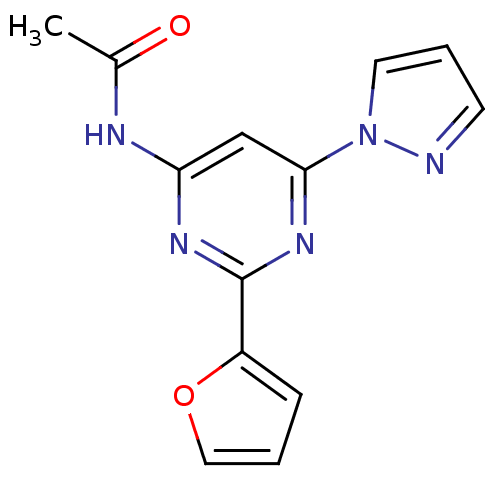
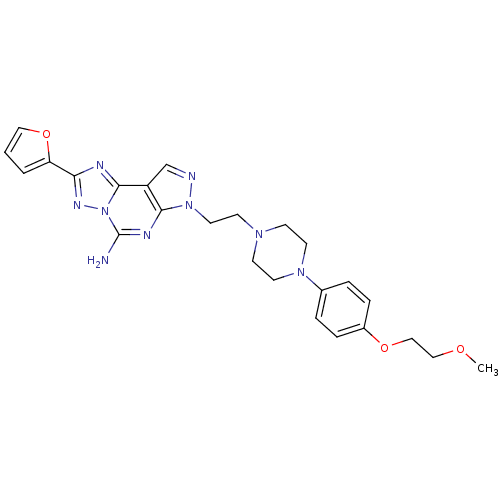

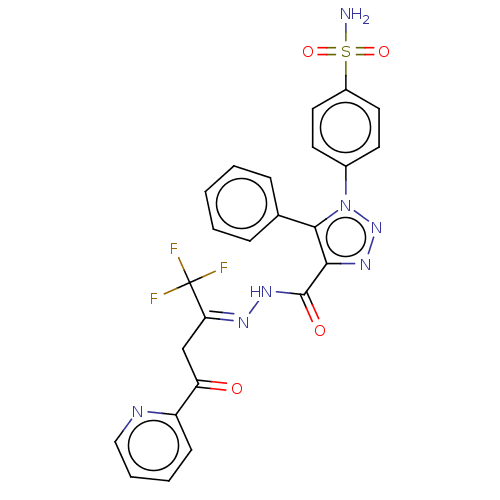
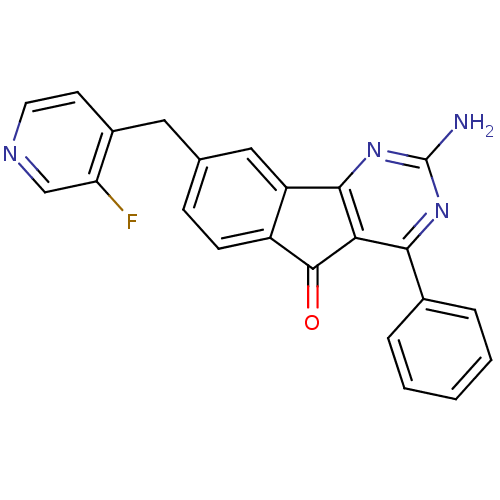
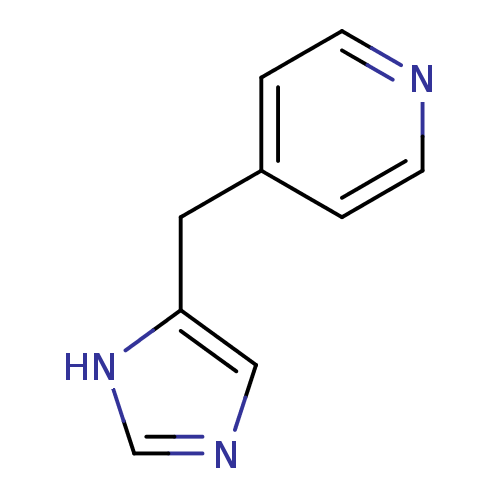
 3D Structure (crystal)
3D Structure (crystal)