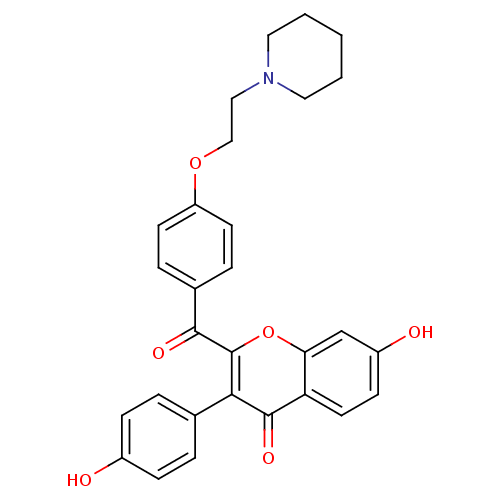
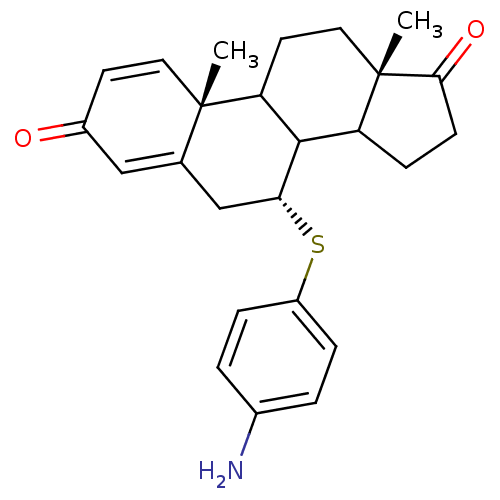
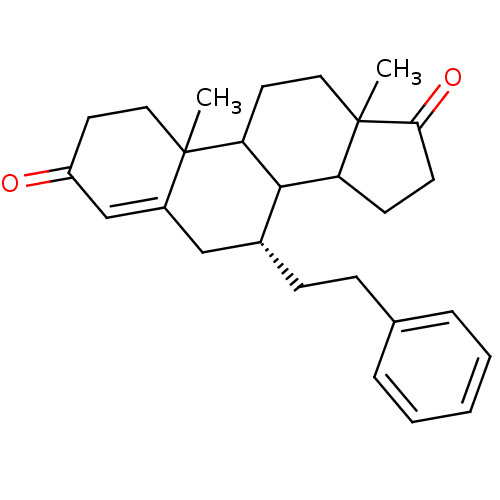
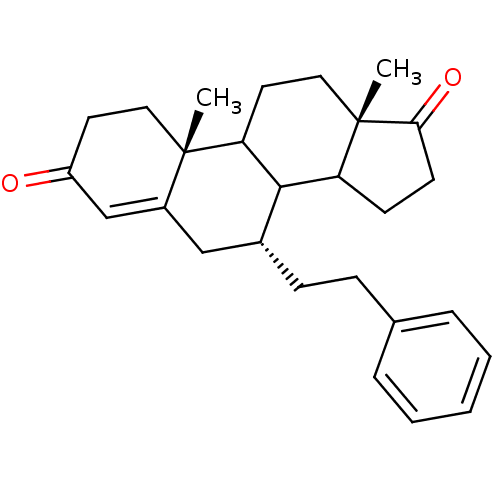
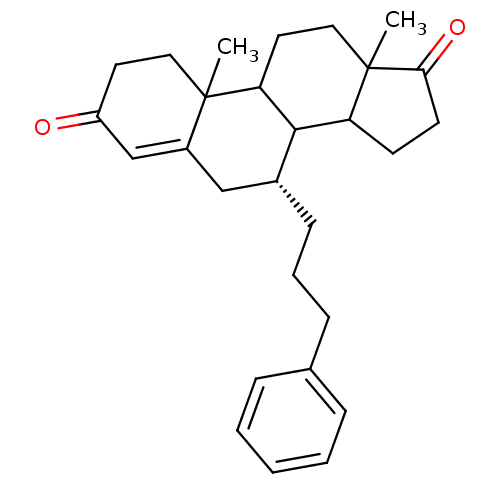
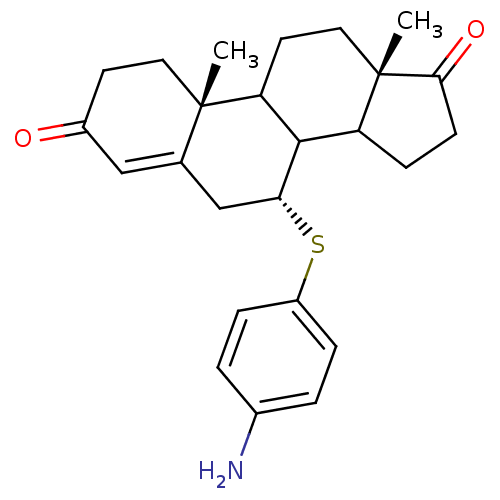
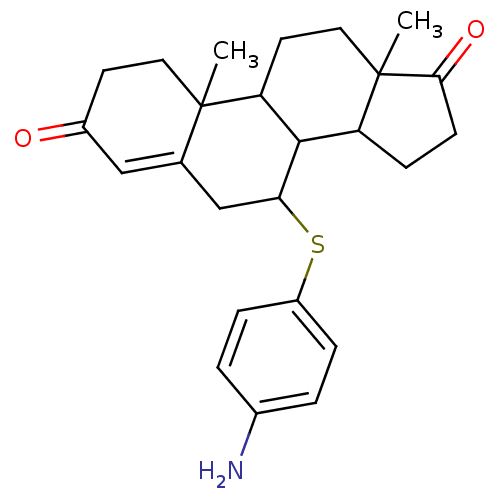
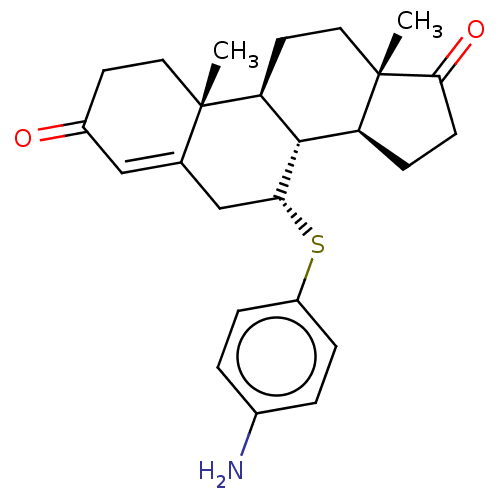
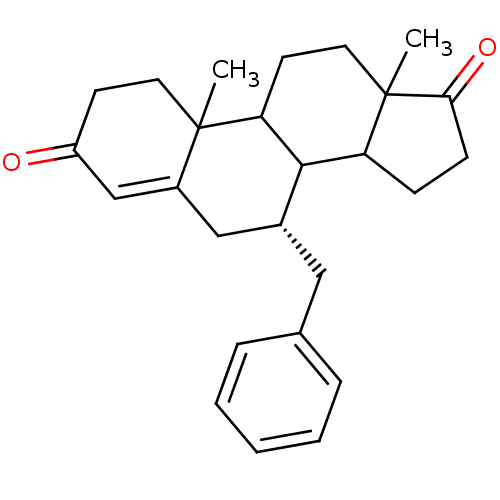
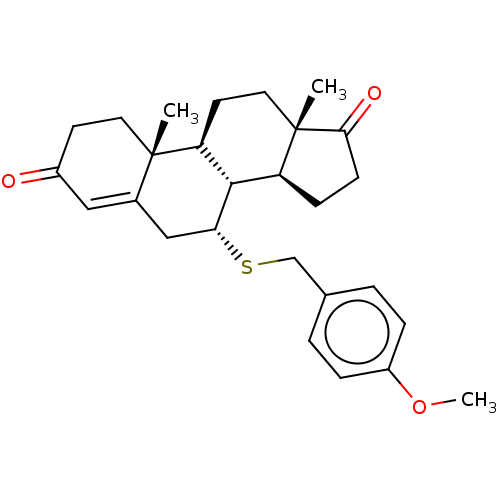
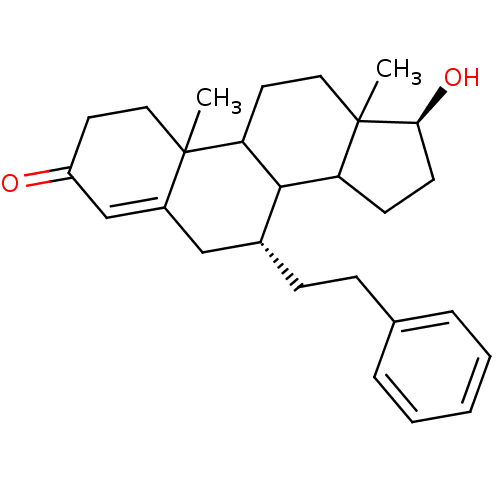
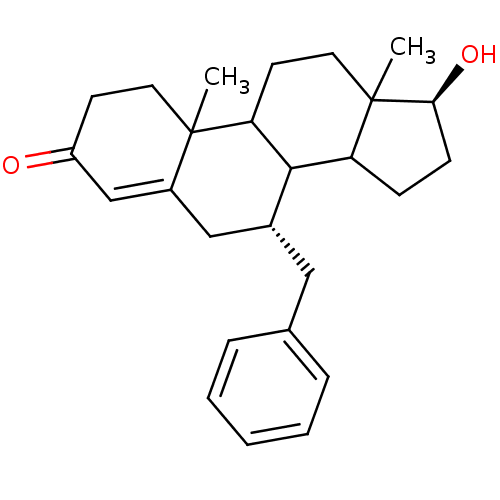
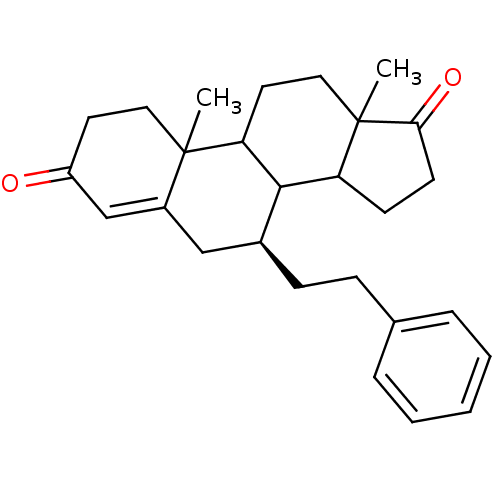
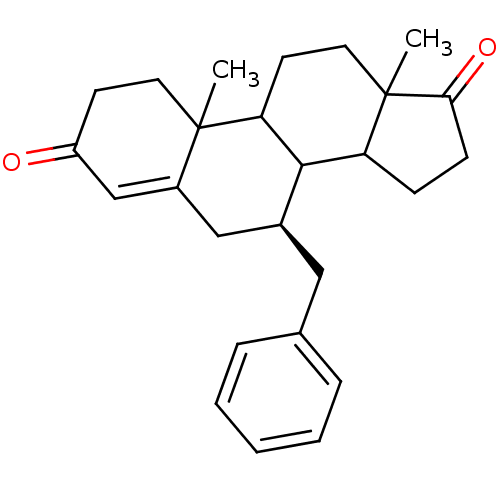
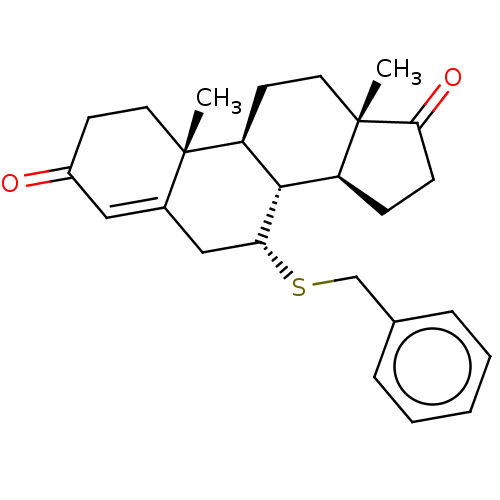
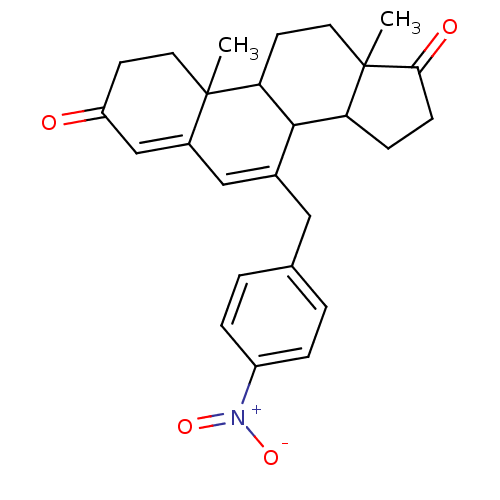
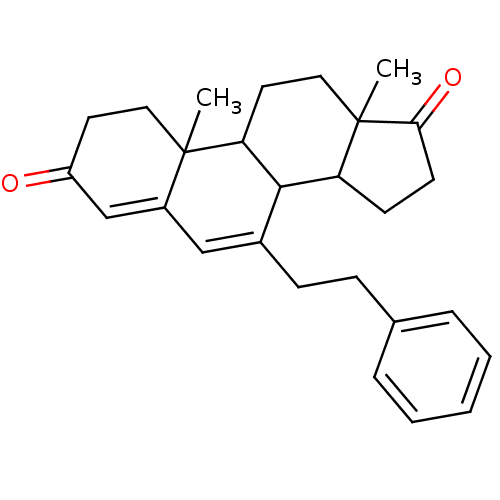
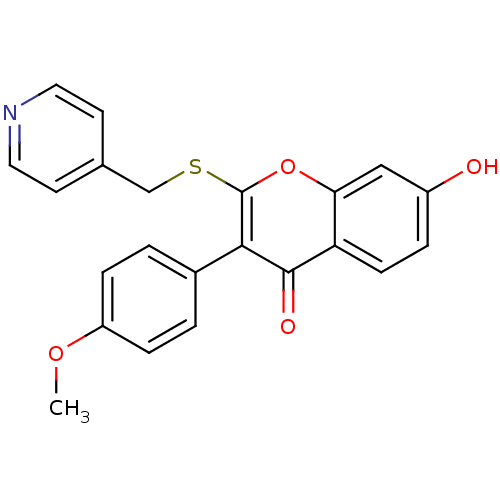
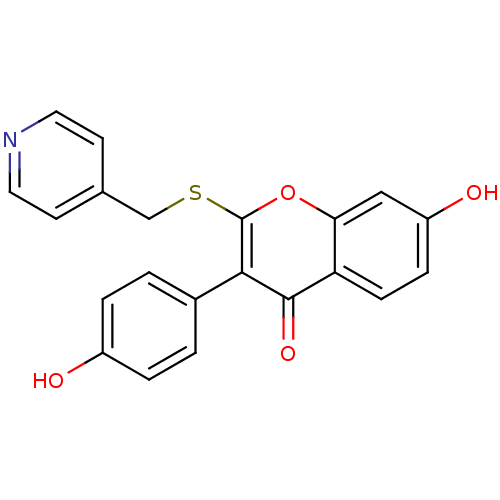
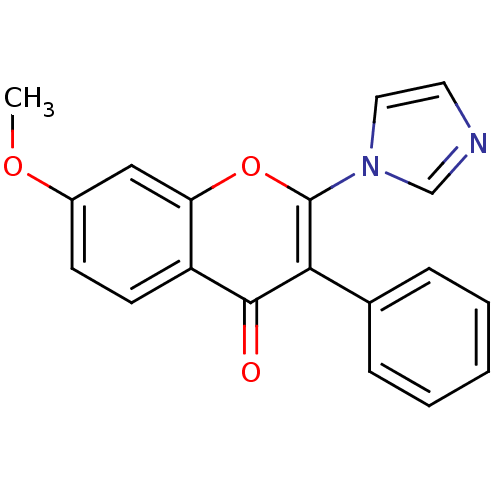
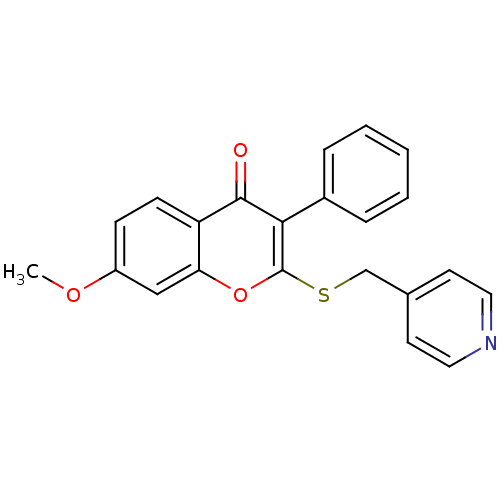
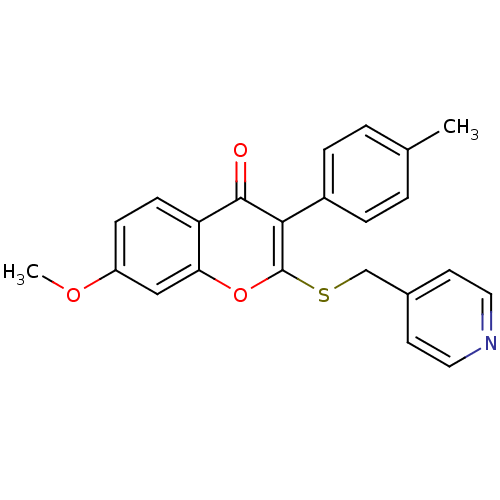
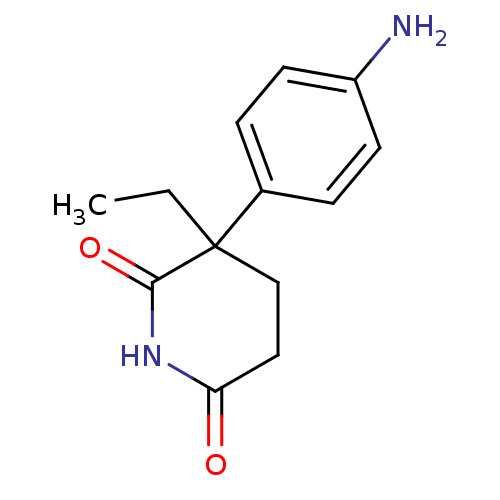
Affinity DataKi: 0.0900nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.290nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.980nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Competitive-inhibition of recombinant human C-terminal His6-tagged Sphk1 expressed in baculovirus infected Sf21 insect cells using varying levels of ...More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Binding affinity for Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: >10nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: >10nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of aromatase cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: >10nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 13.1nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 13.1nMAssay Description:Inhibition of aromatase cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 16.5nMAssay Description:Inhibition of aromatase cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 16.5nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of aromatase cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene...More data for this Ligand-Target Pair
Affinity DataKi: 18.9nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: >20nMAssay Description:Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene...More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 39nM ΔG°: -44.0kJ/mole IC50: 79nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 39.5nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 40.2nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 44.5nMAssay Description:Apparent binding affinity for human placental Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene...More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene...More data for this Ligand-Target Pair
Affinity DataKi: 69nMAssay Description:Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene...More data for this Ligand-Target Pair
Affinity DataKi: 74nM ΔG°: -42.3kJ/mole IC50: 90nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 88nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 88nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 94nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 95nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 114nM ΔG°: -41.2kJ/mole IC50: 112nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 174nMAssay Description:In vitro inhibition of human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 220nM ΔG°: -39.5kJ/mole IC50: 210nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 250nM ΔG°: -39.2kJ/mole IC50: 520nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 260nM ΔG°: -39.1kJ/mole IC50: 220nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 310nM ΔG°: -38.6kJ/mole IC50: 280nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 680nM ΔG°: -36.6kJ/mole IC50: 770nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 900nM ΔG°: -35.9kJ/mole IC50: 1.60E+3nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.16E+3nM ΔG°: -35.2kJ/mole IC50: 3.00E+3nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.41E+3nM ΔG°: -34.7kJ/mole IC50: 2.80E+3nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.41E+3nM ΔG°: -34.7kJ/mole IC50: 2.80E+3nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.41E+3nM ΔG°: -34.7kJ/mole IC50: 2.80E+3nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair




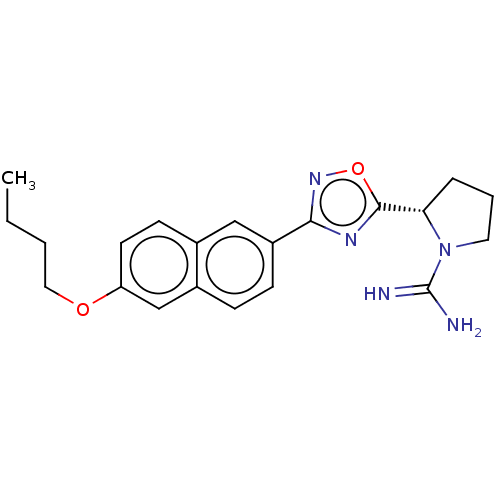

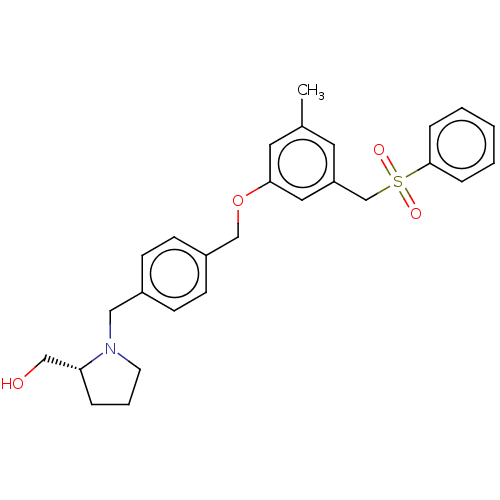
 3D Structure (crystal)
3D Structure (crystal)