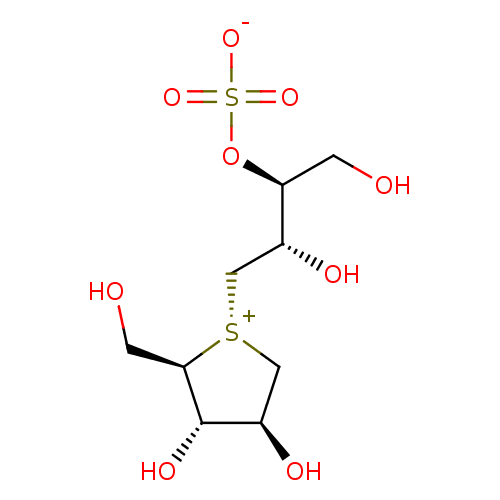
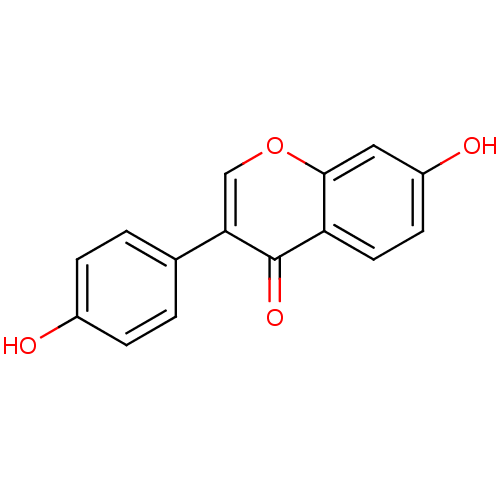
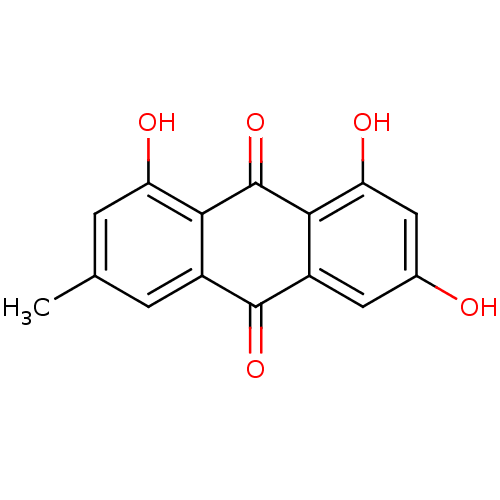
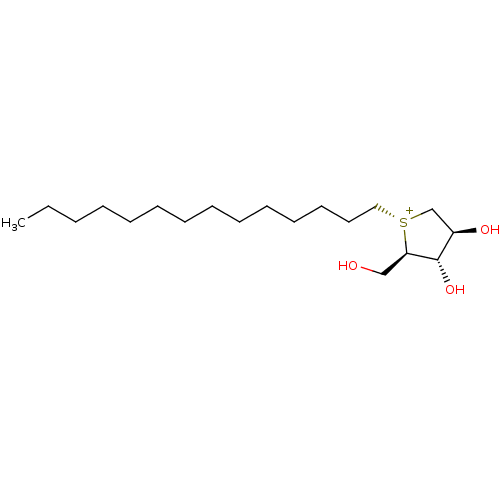
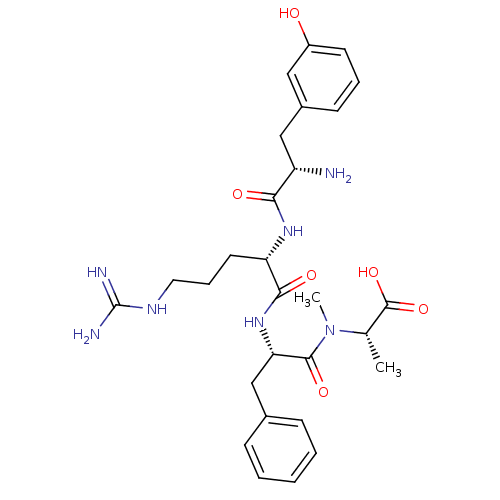
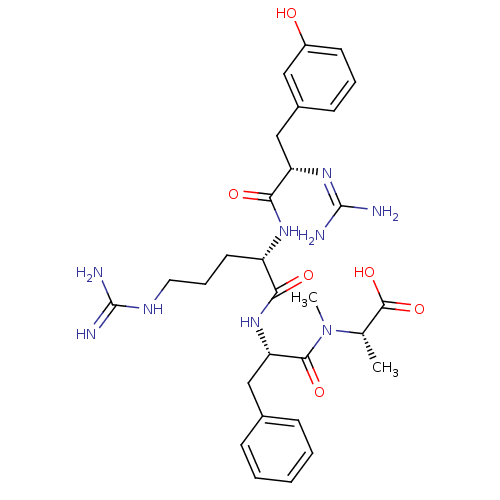
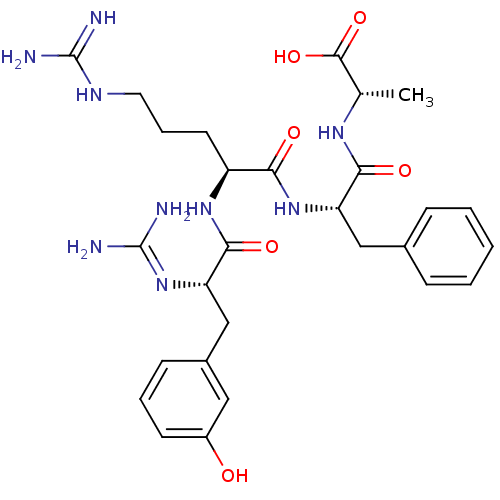
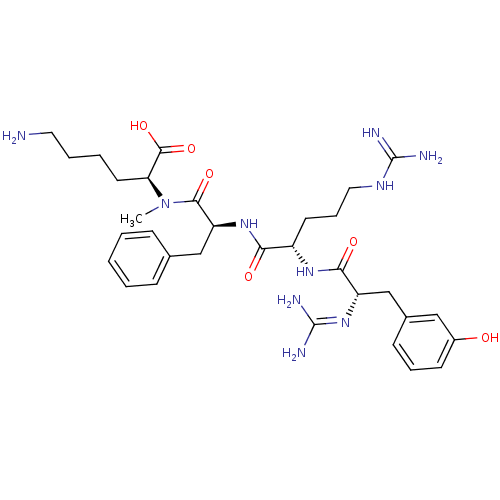
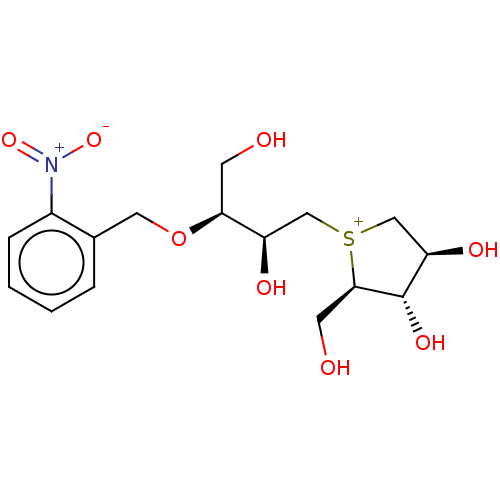
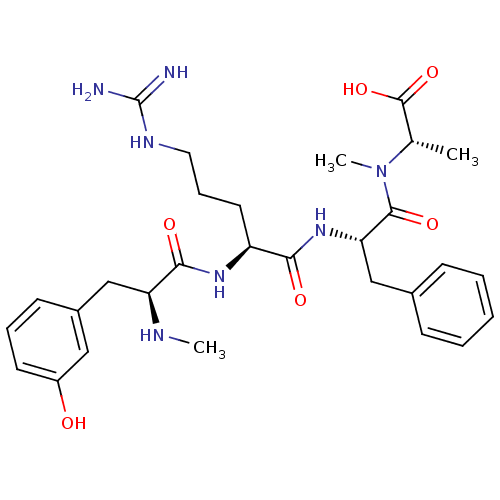
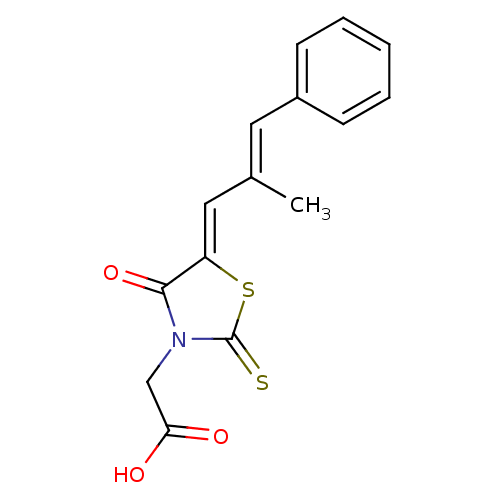
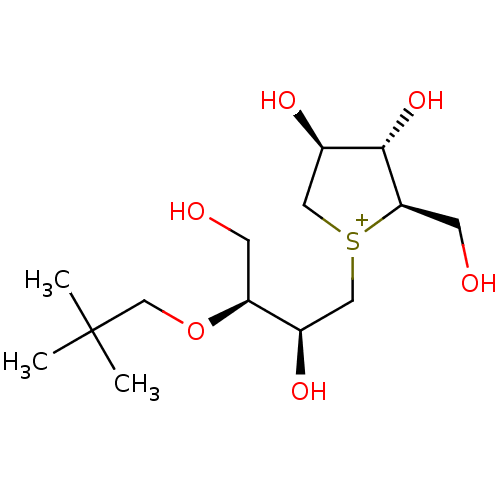
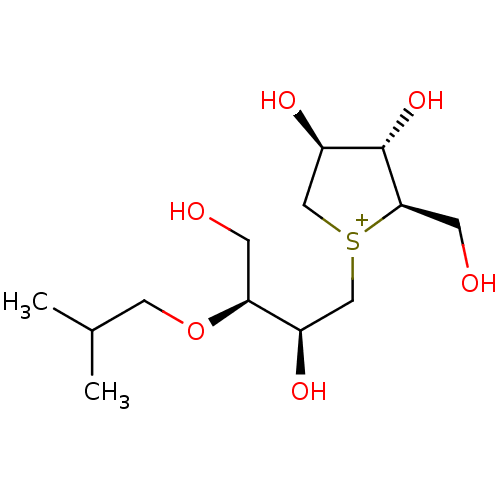
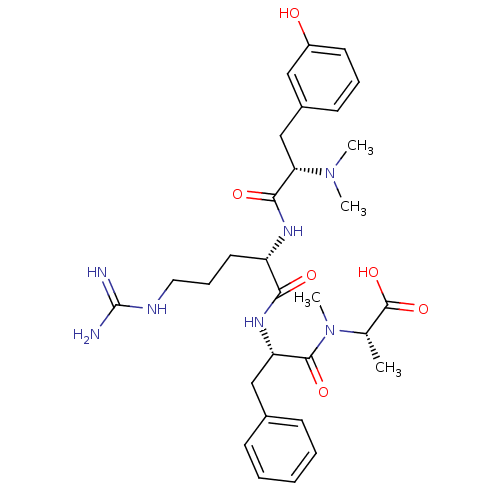
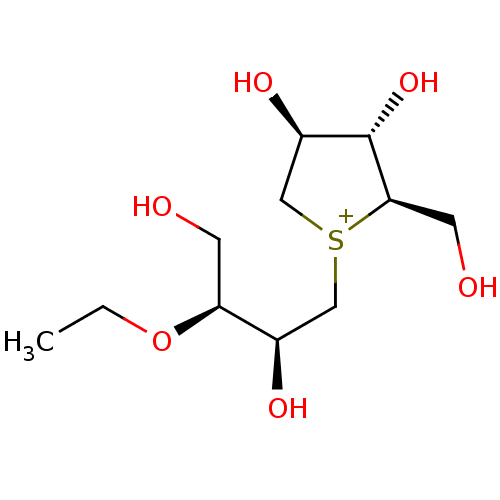
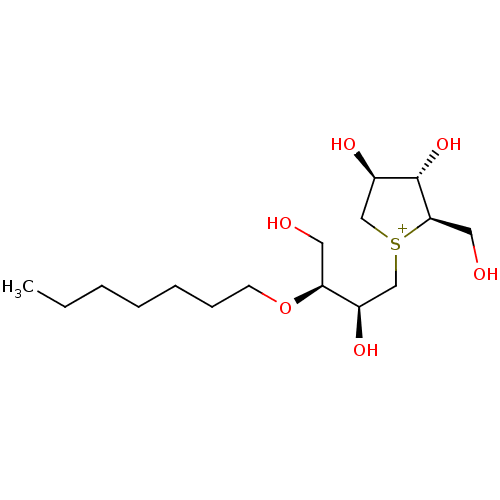
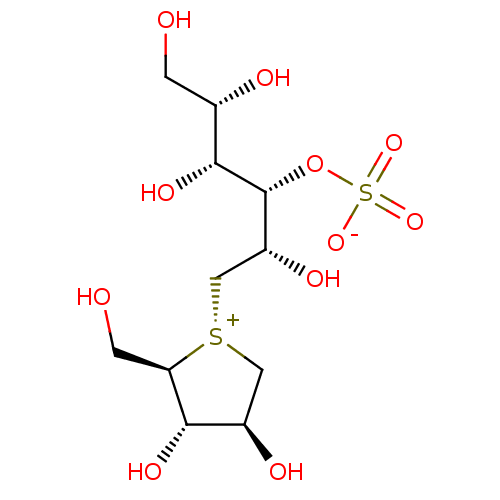
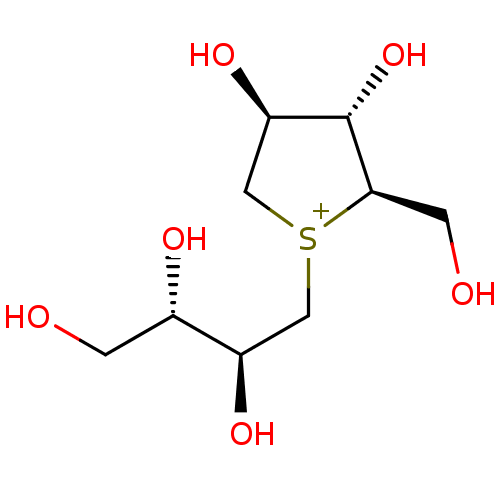
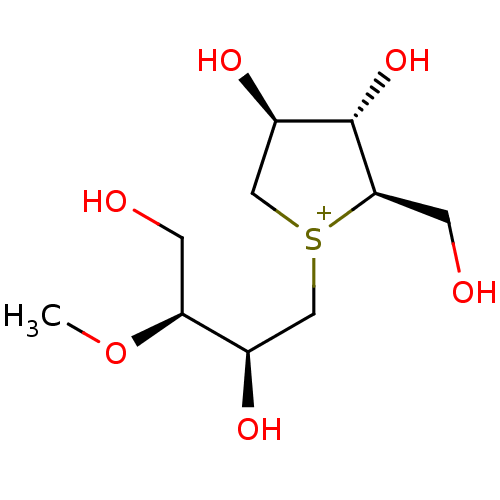
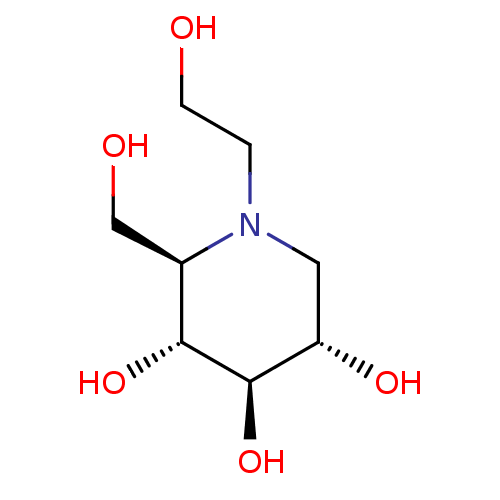
Affinity DataKi: 24nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 370nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 770nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 5.70E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nMAssay Description:Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla...More data for this Ligand-Target Pair
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla...More data for this Ligand-Target Pair
Affinity DataIC50: 10.2nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
Affinity DataIC50: 12.9nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
Affinity DataIC50: 20.8nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
Affinity DataIC50: 32.9nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Inhibition of aldose reductase in rat lens homogenates by fluorophotometerMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Inhibition of rat lens aldose reductase using DL-glyceraldehyde as substrate after 30 mins by fluorescence microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 101nMAssay Description:Binding affinity of the compound towards Opioid receptor mu 1 by the displacement of [3H]-DAMGO in mouse spinal cordMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 640nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 950nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)