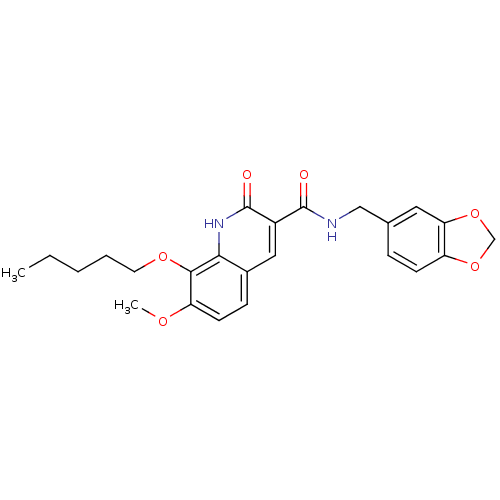
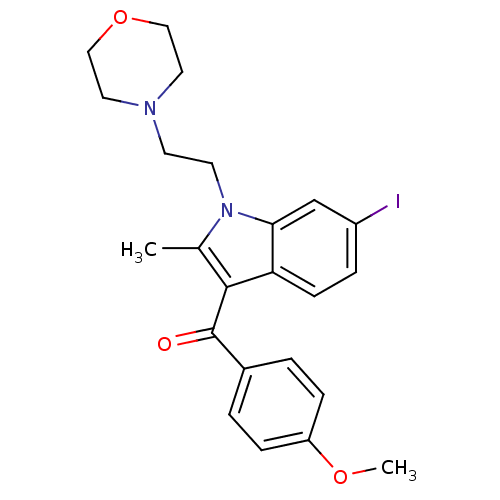
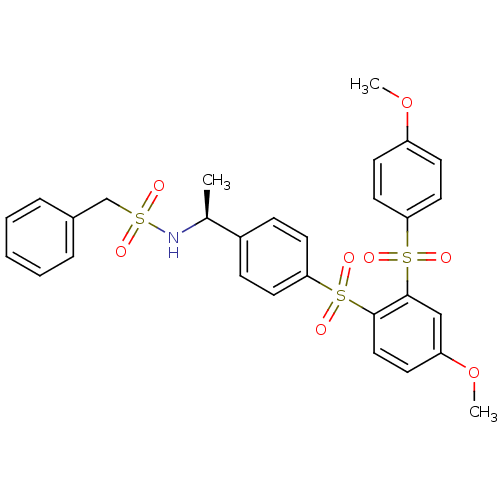
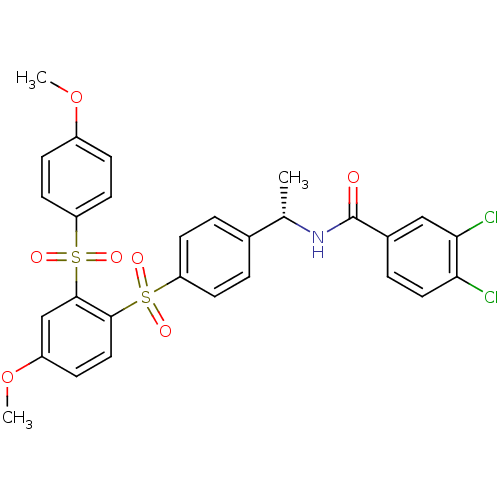
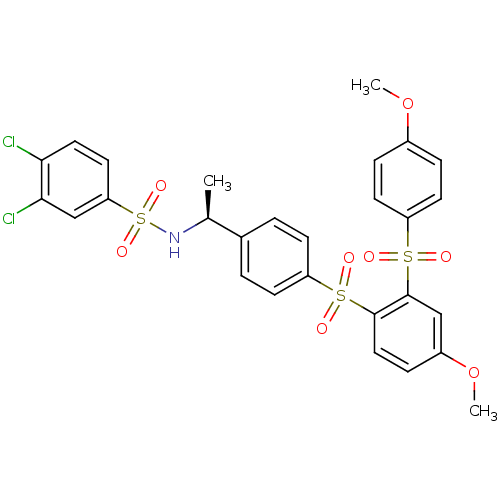
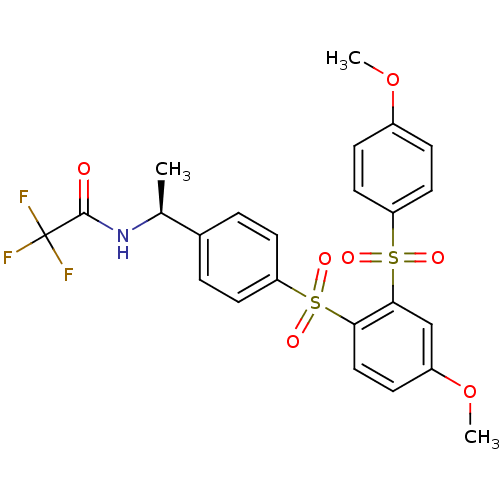
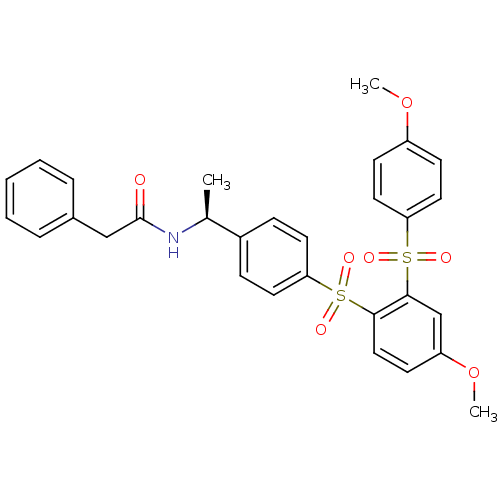
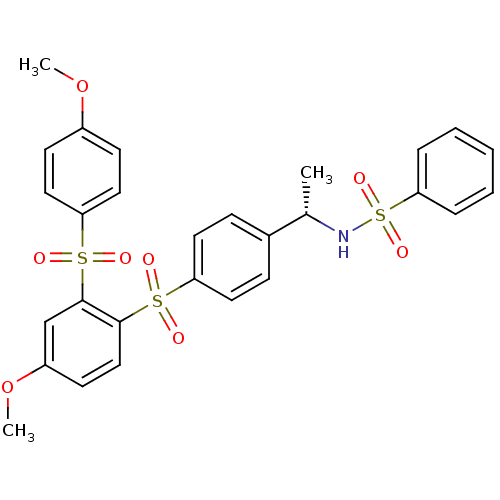
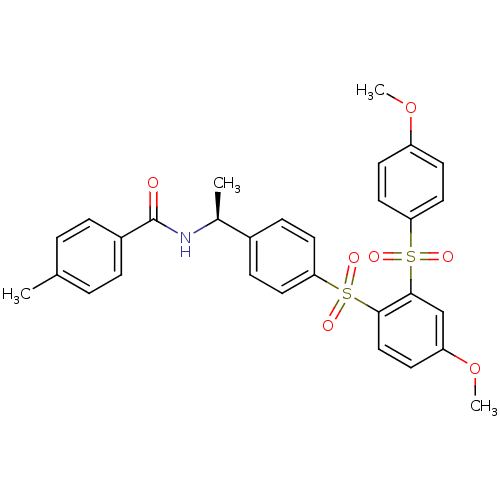
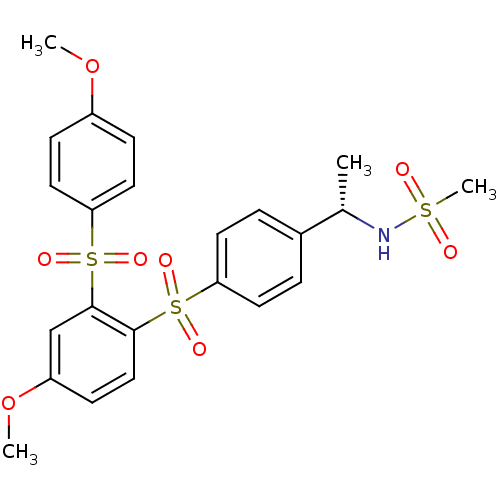
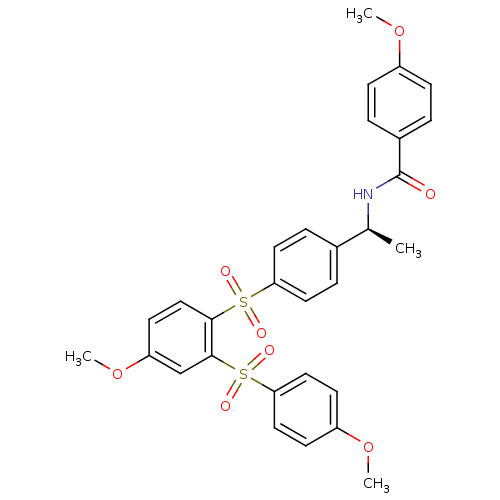
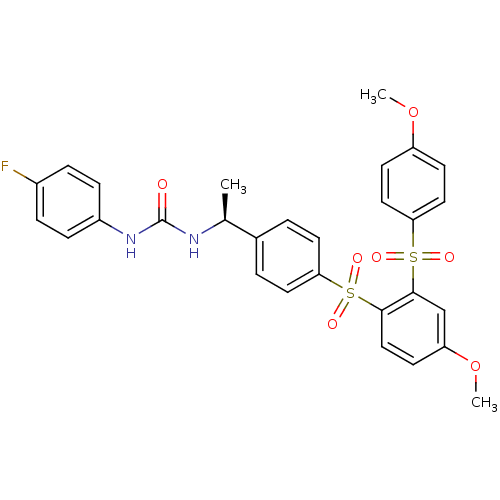
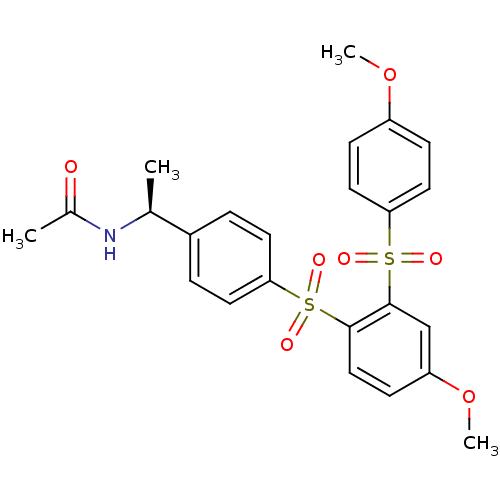
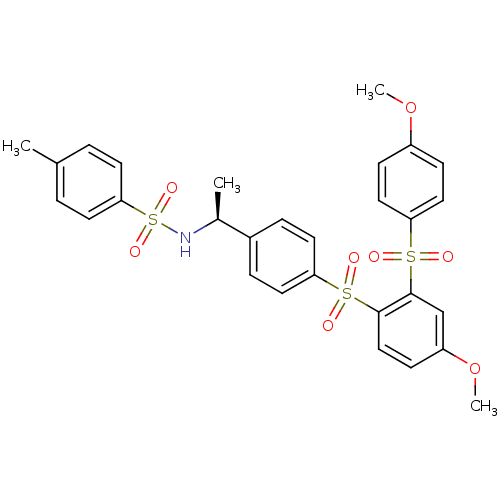
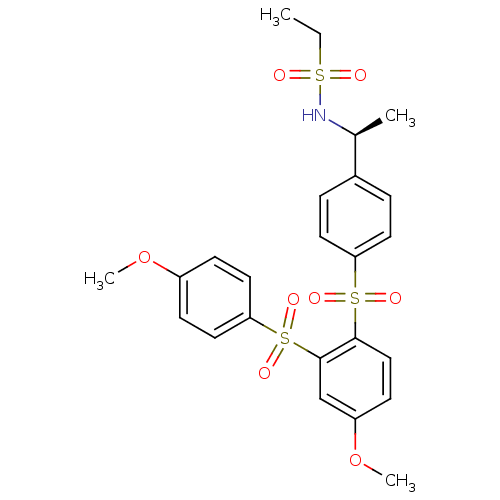
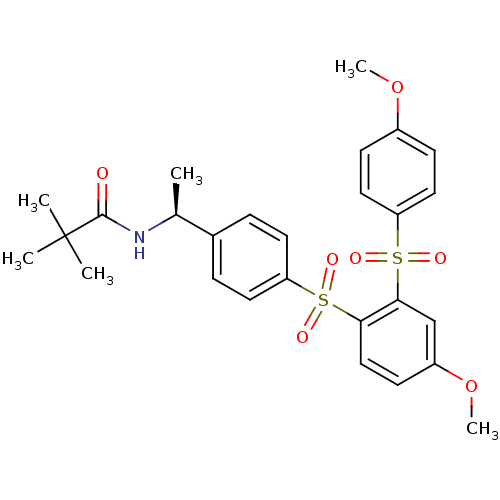
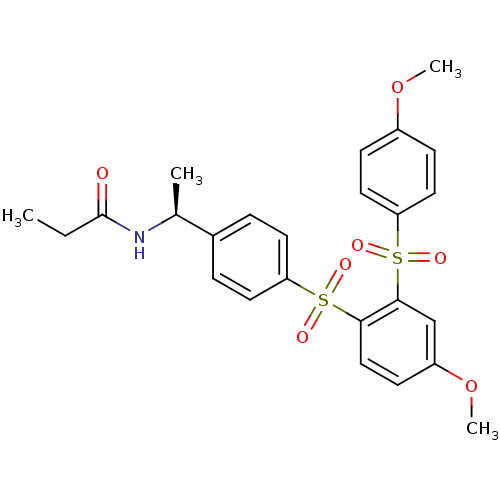
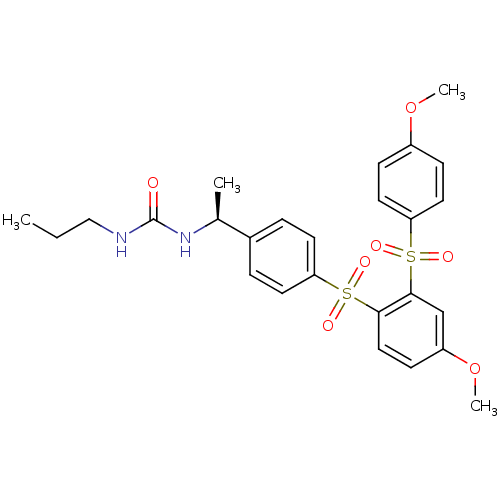
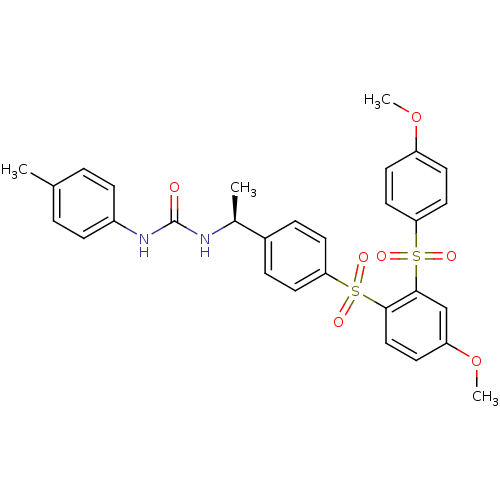
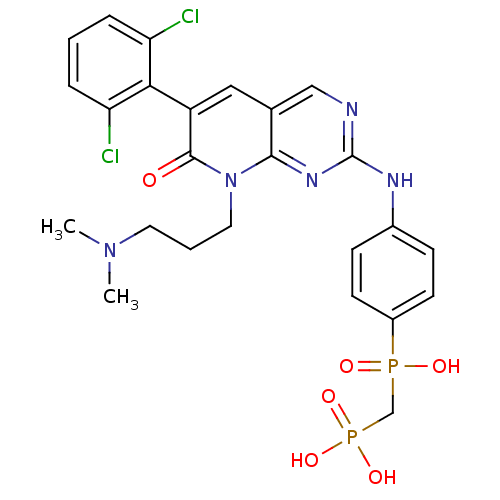
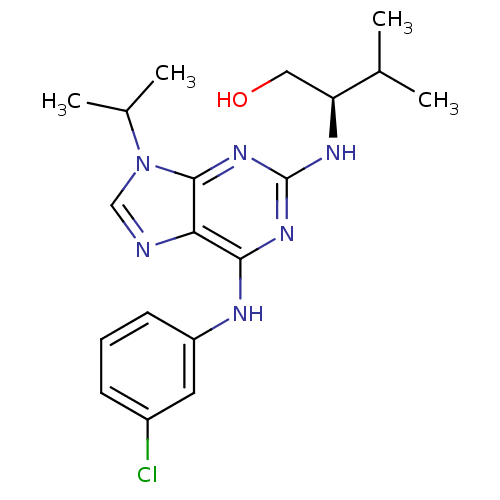
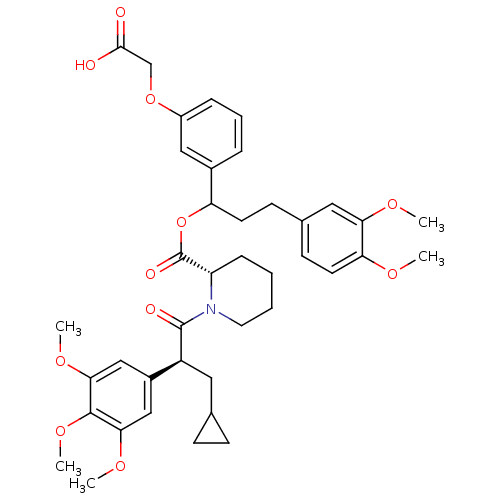
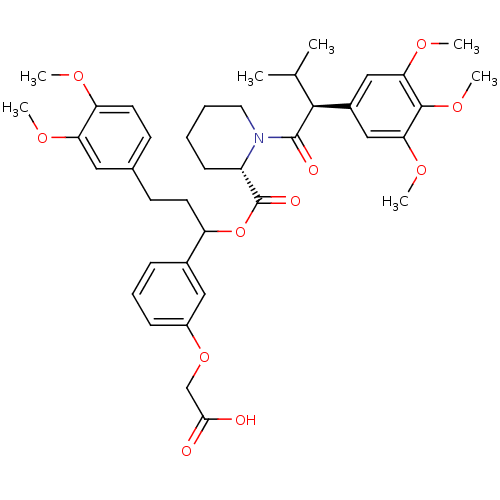
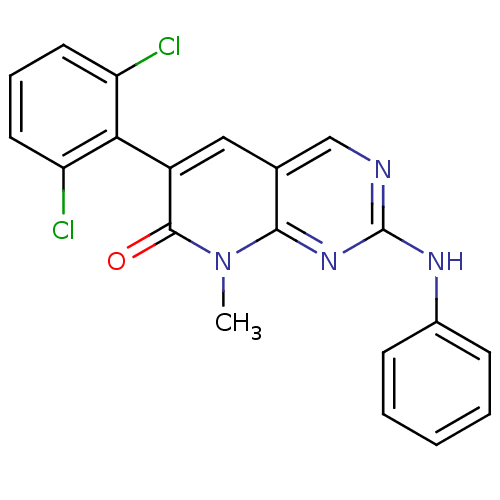
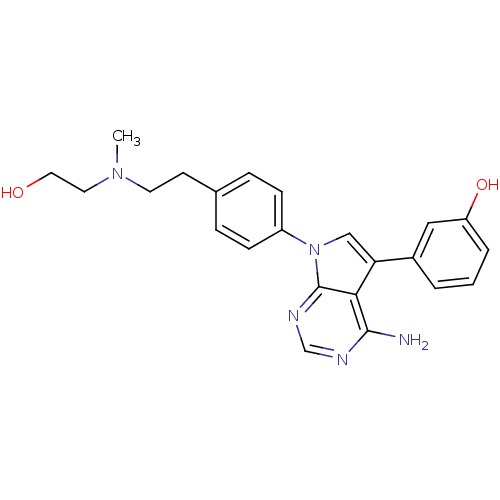
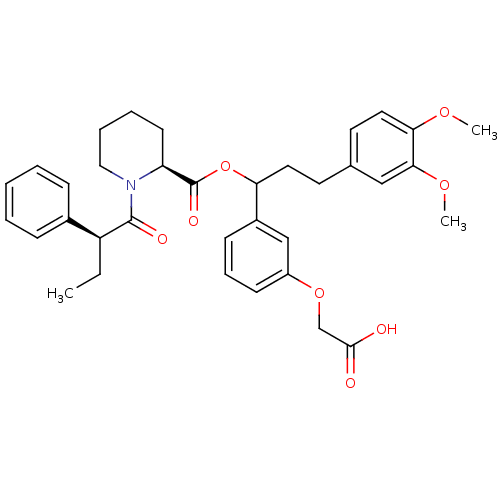
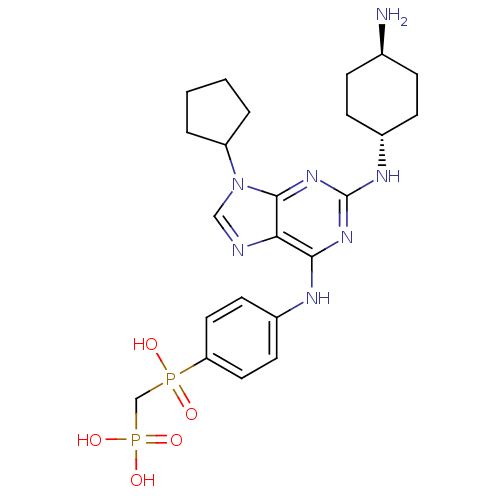
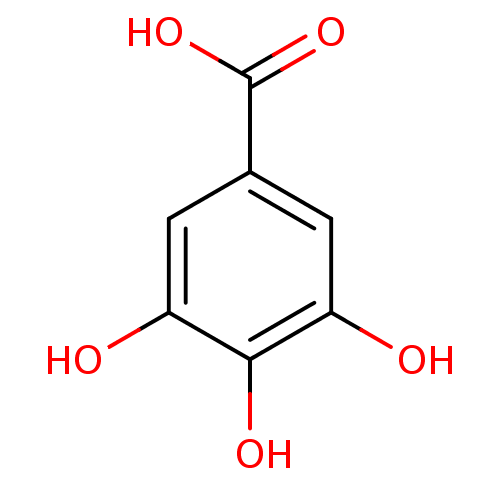
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Binding affinity for cannabinoid receptor 2More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 31.2nMAssay Description:Binding affinity for human cannabinoid receptor 2 was determined by using [3H]-CP-55,940 as radioligandMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 35.9nMAssay Description:Binding affinity for cannabinoid receptor 2More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 35.9nMAssay Description:Binding affinity for cannabinoid receptor 2More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 79nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 84nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 117nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 235nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 478nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 855nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 884nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 905nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.13E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.17E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.17E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.76E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.78E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.92E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.97E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.31E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 3.56E+3nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.06E+4nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 7.21E+4nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.00E+5nMAssay Description:Binding affinity for cannabinoid receptor 1More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.450nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 26nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assayMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
Affinity DataIC50: 60nMpH: 7.3 T: 2°CAssay Description:A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair