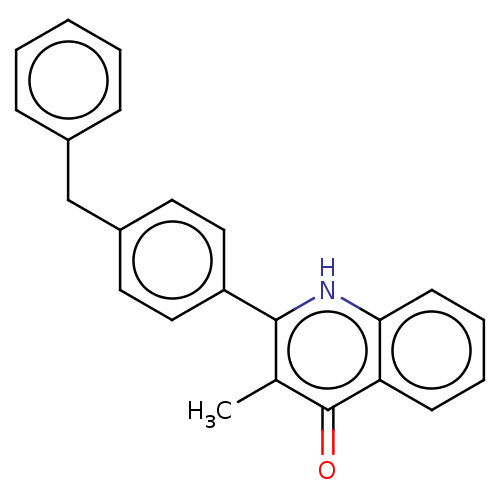
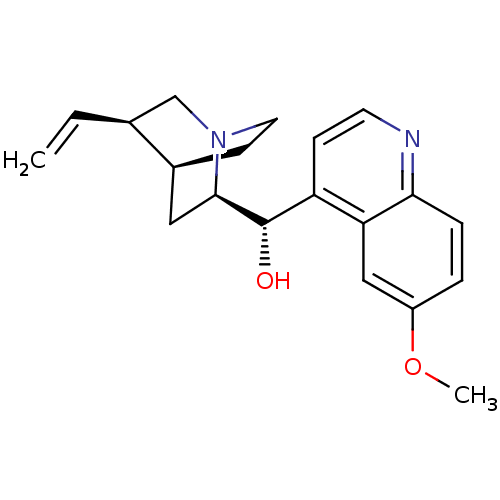
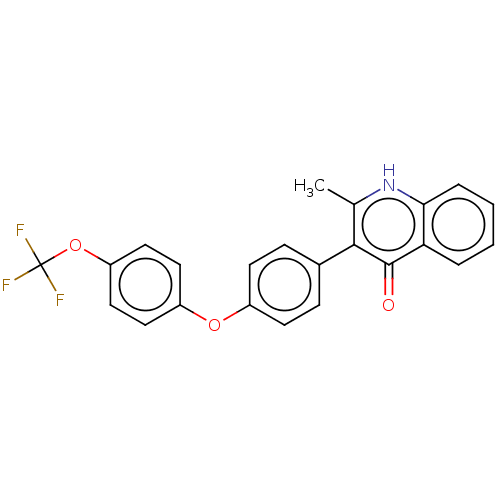
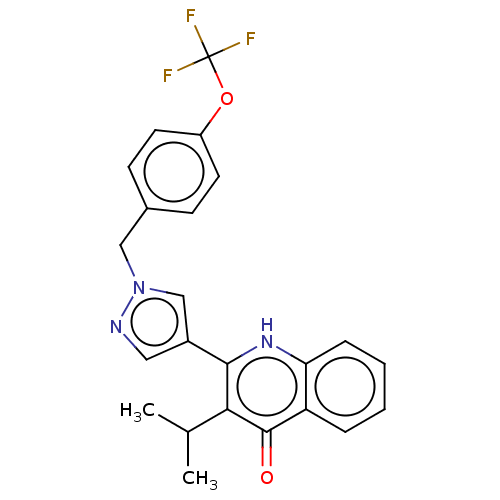
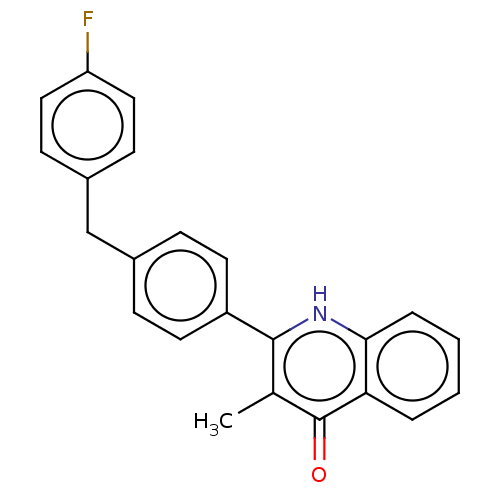
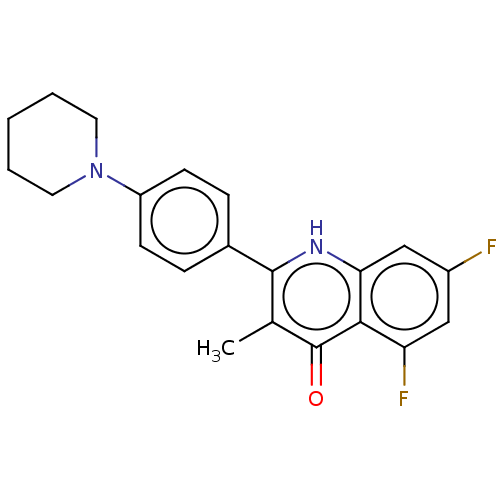
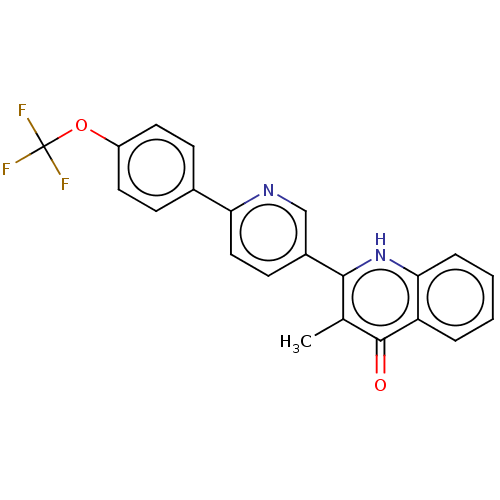
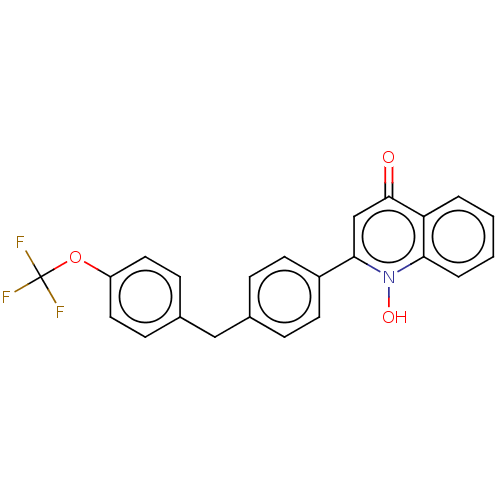
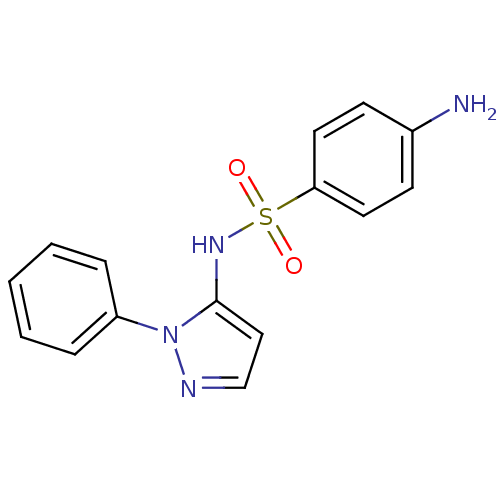
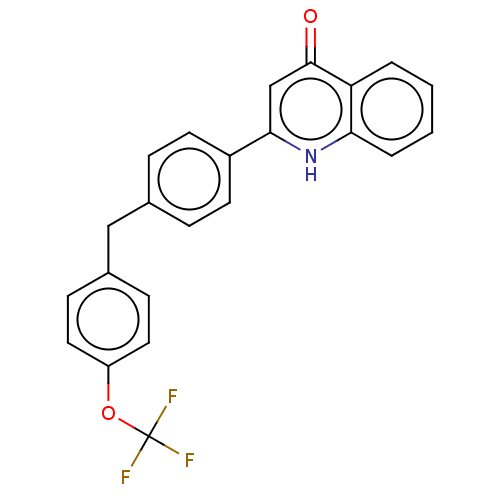
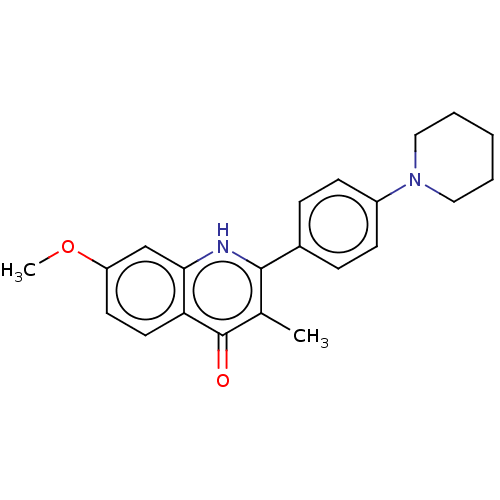
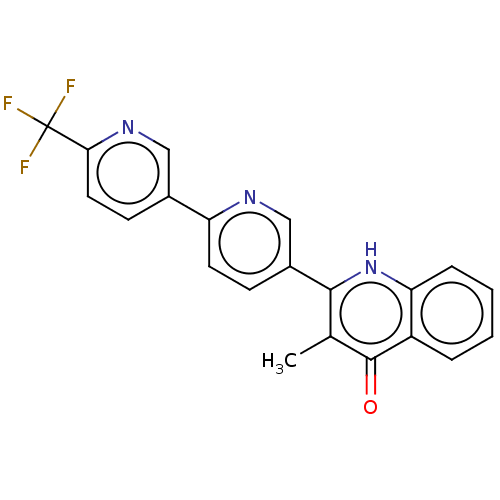
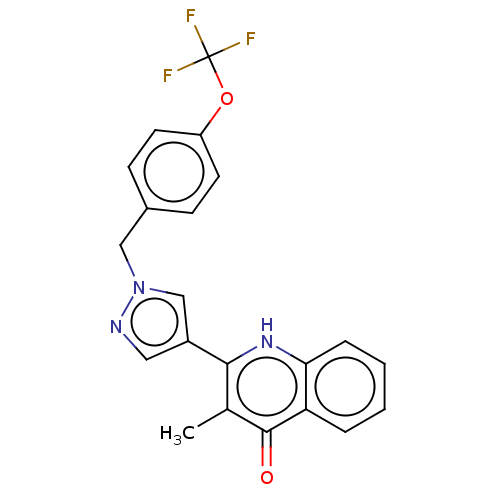
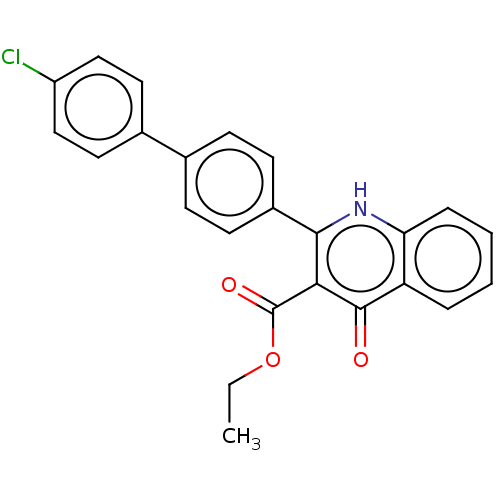
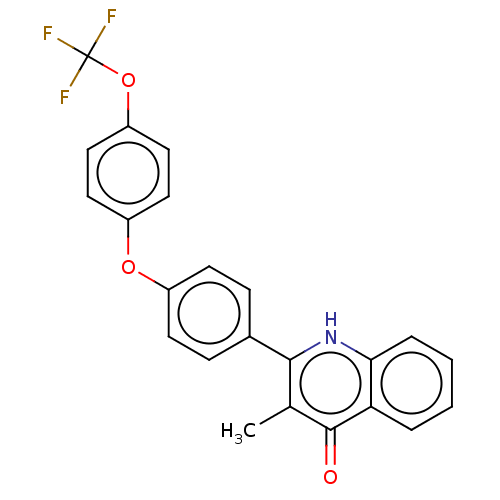
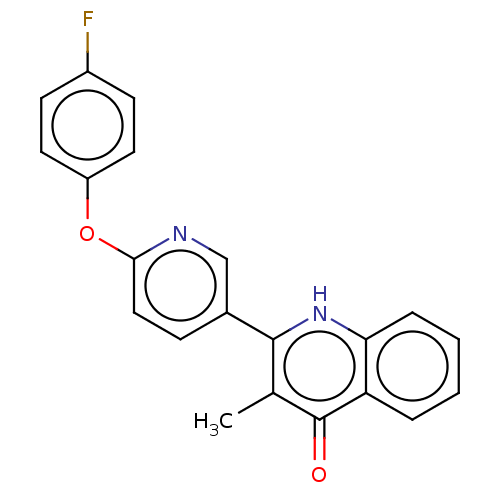
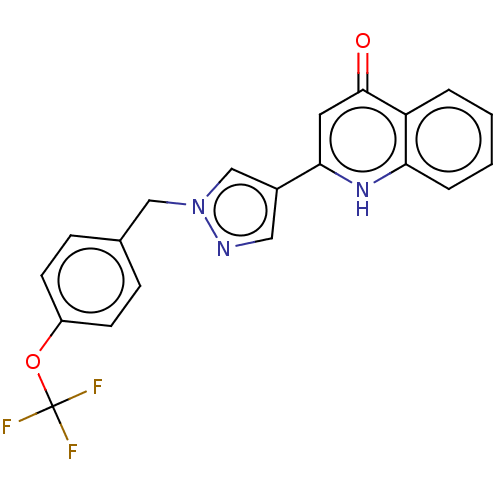
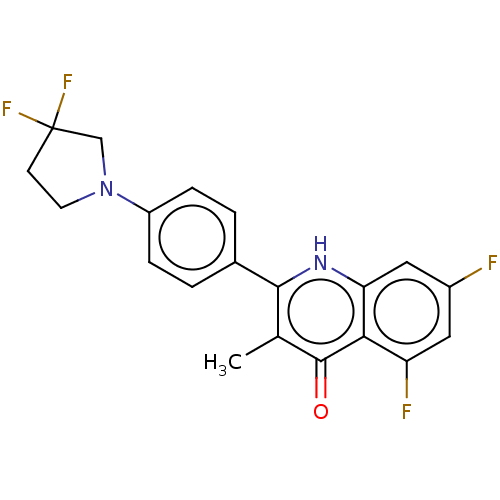
Affinity DataIC50: 9nMAssay Description:Inhibition of DPP4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 20nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 70nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 82nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 100nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 140nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 250nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 270nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 290nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 310nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 380nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 440nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 750nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 1.40E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 2.02E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 3.60E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 3.60E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 4.47E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 6.50E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 6.50E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 6.50E+3nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 1.06E+4nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
TargetProbable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)()
TBA
US Patent
TBA
US Patent
Affinity DataIC50: 1.58E+4nMAssay Description:The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of HSL (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of HSL (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+7nMAssay Description:Inhibition of human AChEMore data for this Ligand-Target Pair


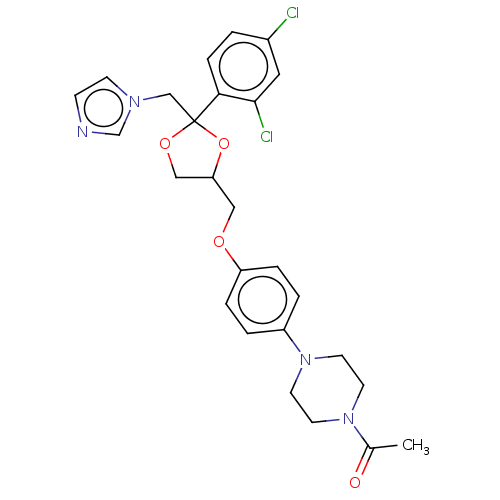
 3D Structure (crystal)
3D Structure (crystal)