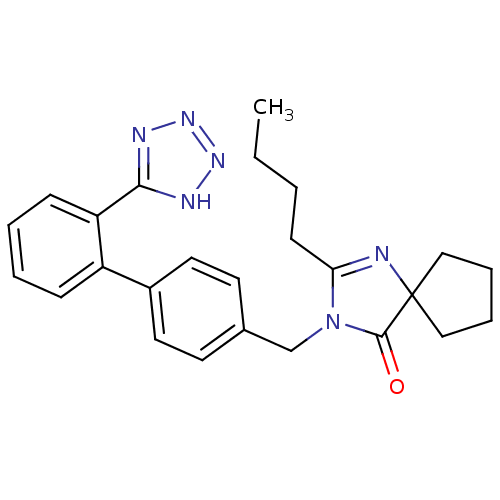
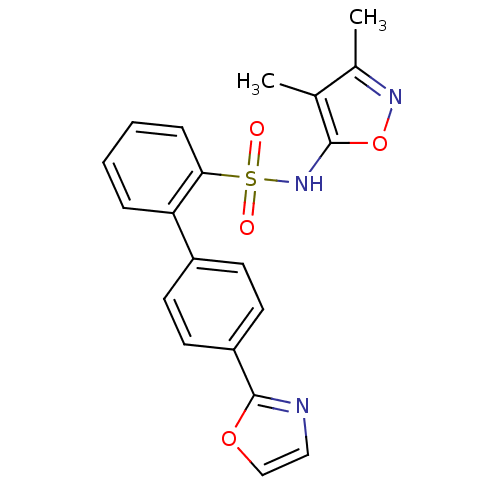
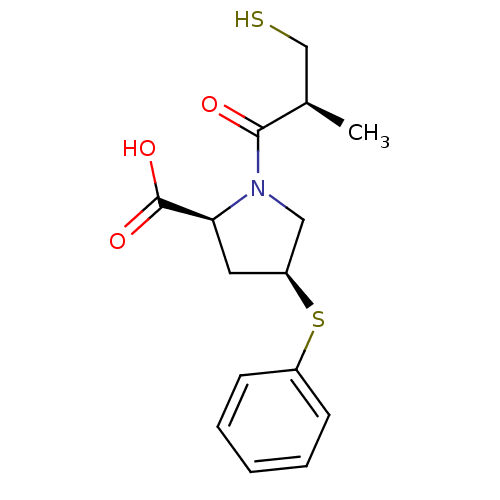
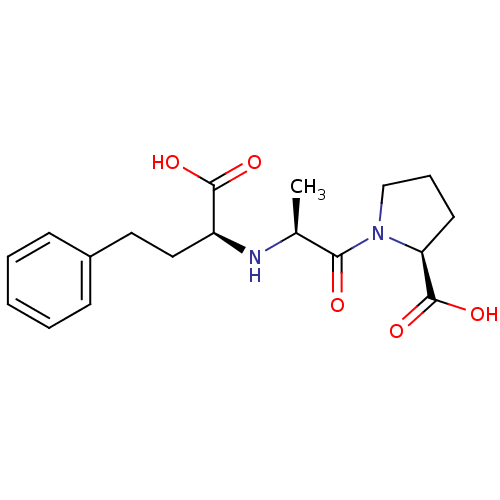
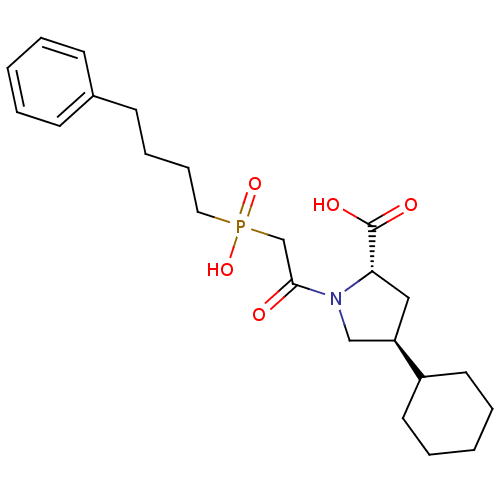
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >0.000100nMAssay Description:Compound was evaluated for its binding affinity towards human Endothelin A receptorMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor B(RAT)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >0.000100nMAssay Description:Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >0.000100nMAssay Description:Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibitory constant against rabbit lung Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor B(RAT)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Compound was evaluated for its binding affinity towards human Endothelin A receptorMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Compound was evaluated for its binding affinity towards human Endothelin A receptorMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor B(RAT)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Receptor binding affinity determined from competitive binding assay using 1251 labelled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor B(RAT)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 4.70nMAssay Description:Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor B(RAT)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Compound was evaluated for its binding affinity towards human Endothelin A receptorMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Compound was evaluated for inhibitory activity against human Angiotensin II receptor, type 1More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Compound was evaluated for inhibitory activity against human Endothelin A receptor (ETA)More data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Compound was evaluated for inhibitory activity against human Endothelin B receptor (ETB)More data for this Ligand-Target Pair