TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 44nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 290nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 369nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 377nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 385nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 440nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 462nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 543nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 720nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.64E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 2.19E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 2.21E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 2.62E+3nMAssay Description:Displacement of radiolabeled MK-499 from human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 2.87E+3nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0700nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 minsMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Antagonist activity at human SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Antagonist activity at mouse SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of cannabinoid CB1 receptorMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o...More data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMAssay Description:Antagonist activity at mouse SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Antagonist activity at mouse SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysisMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o...More data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Displacement of [125I]SS-14 from mouse SSTR3 expressed in CHO cells by TopCount analyzerMore data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Displacement of [125I]SS-14 from mouse SSTR3 expressed in CHO cells by TopCount analyzerMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.450nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 minsMore data for this Ligand-Target Pair

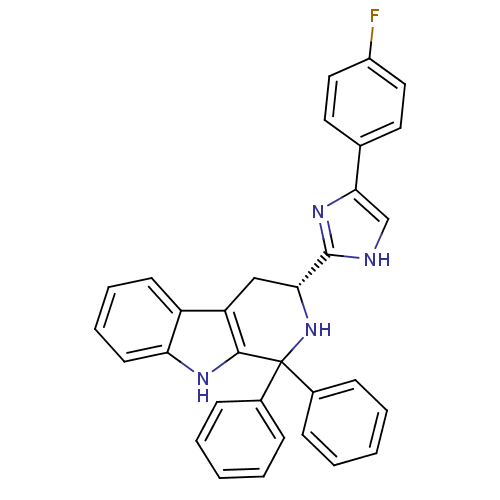
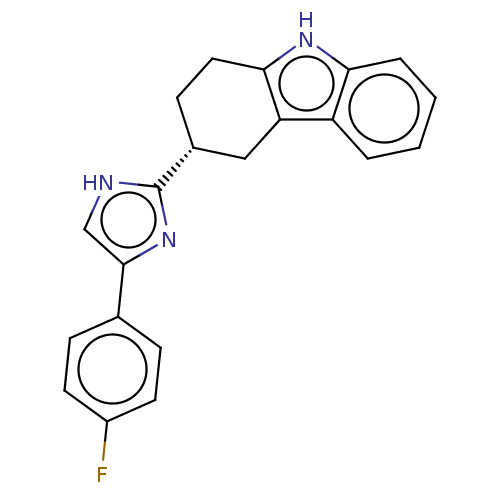
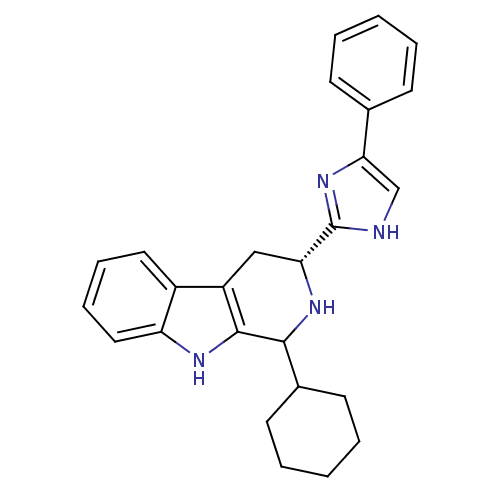


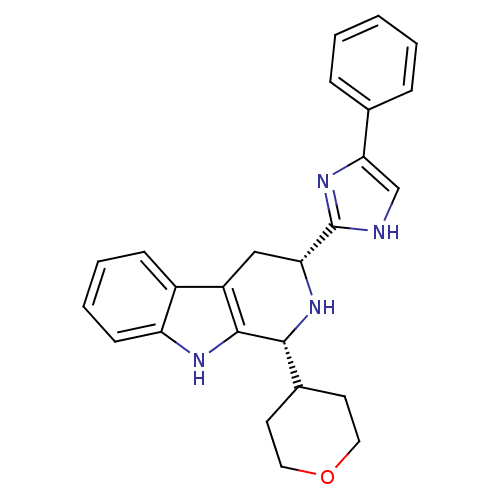
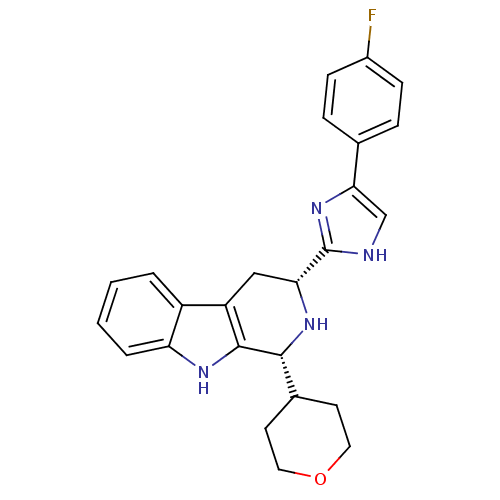


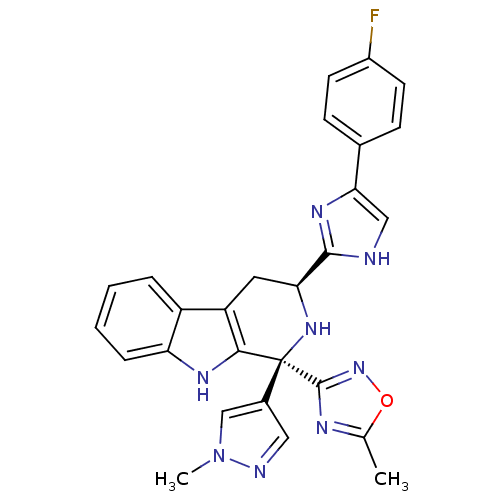
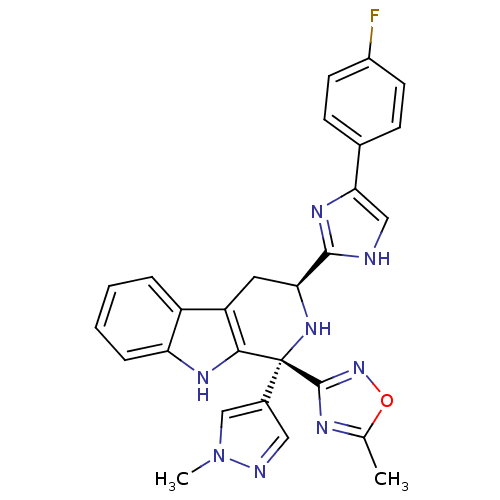
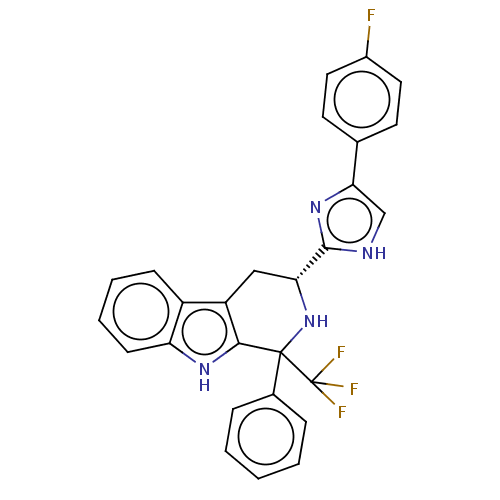
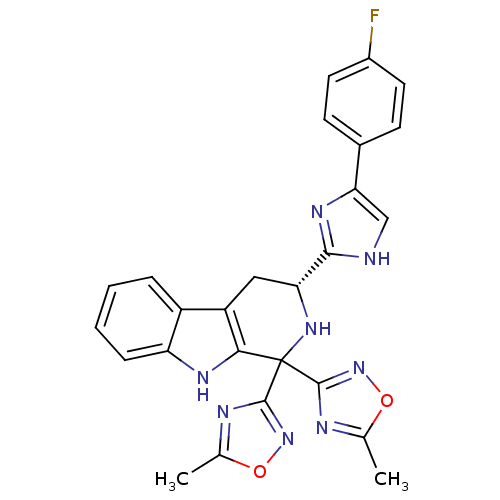

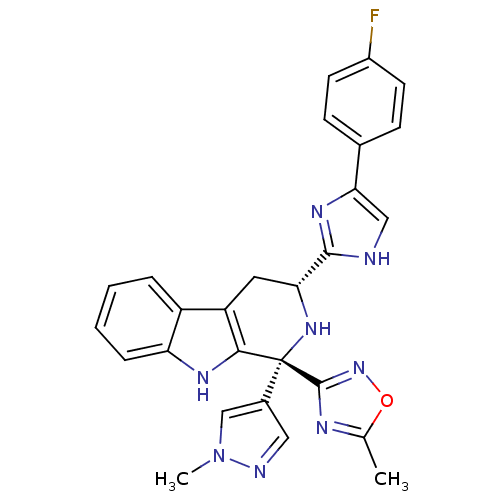




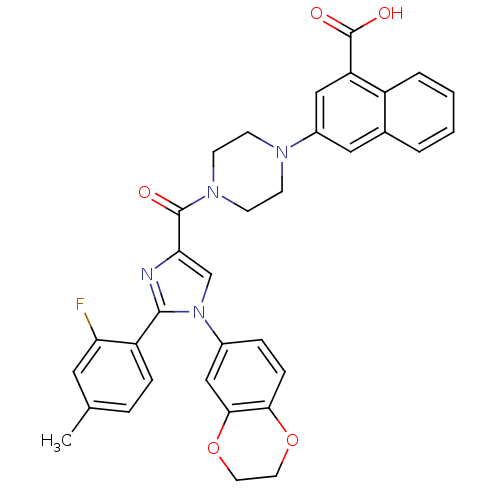

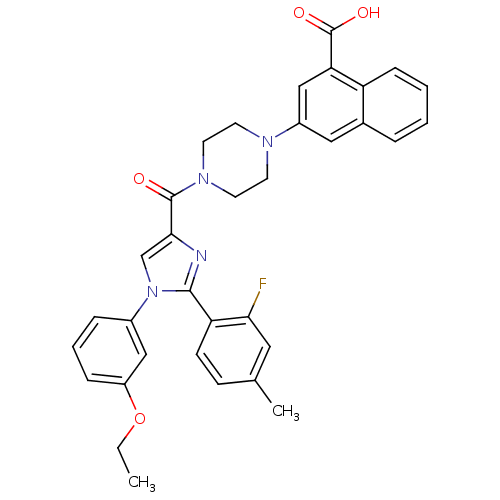
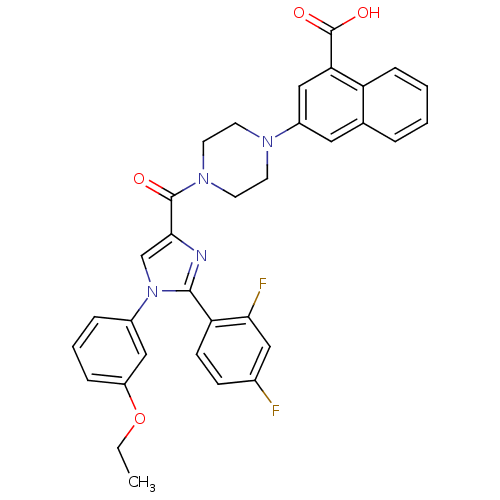
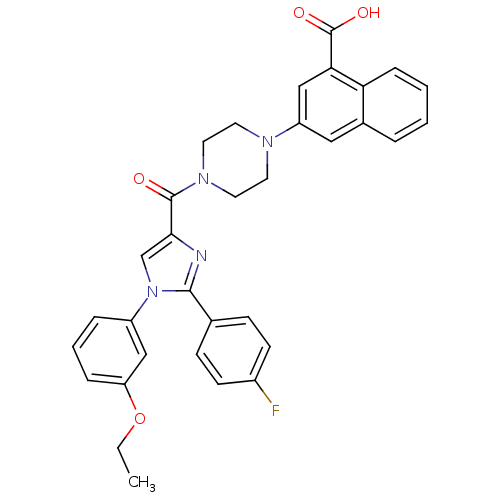


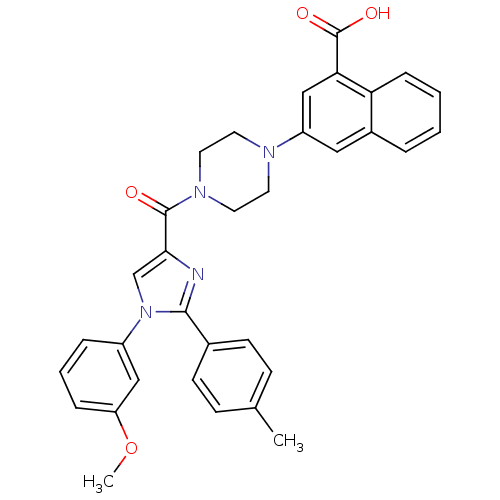
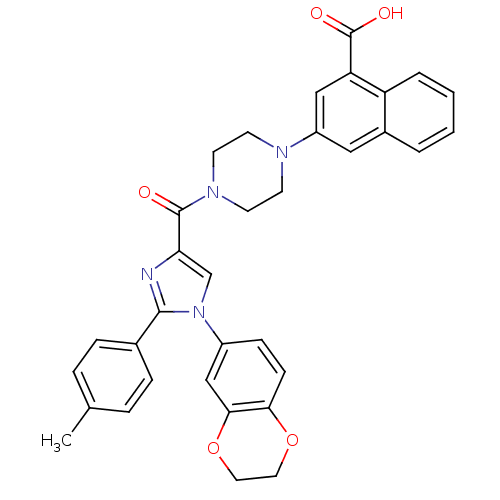


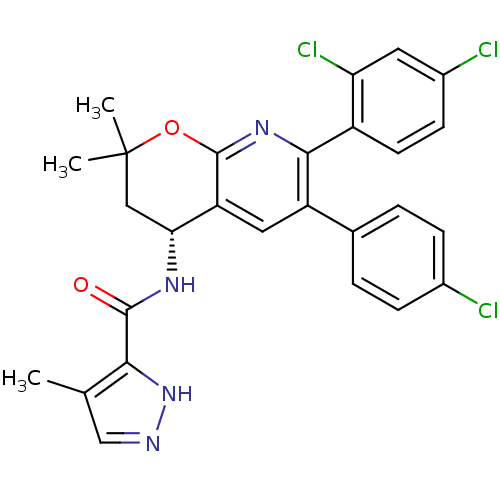
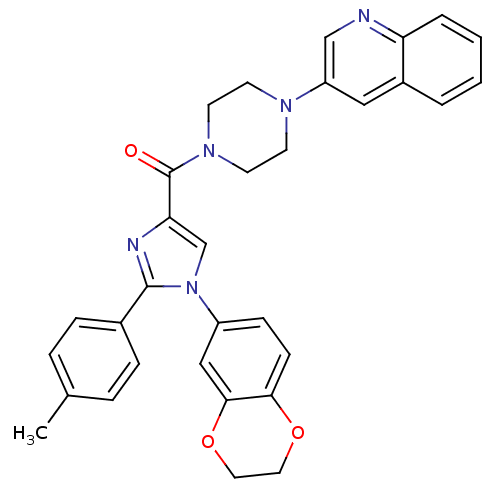
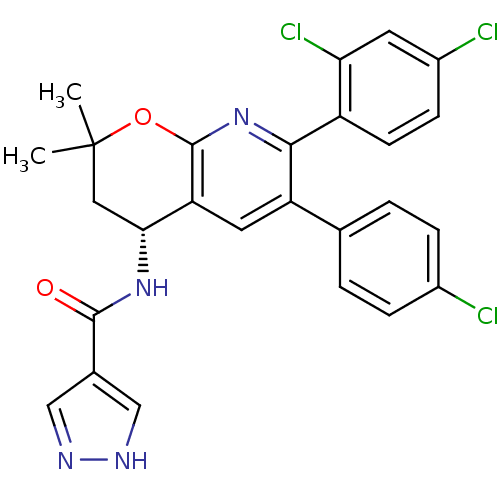
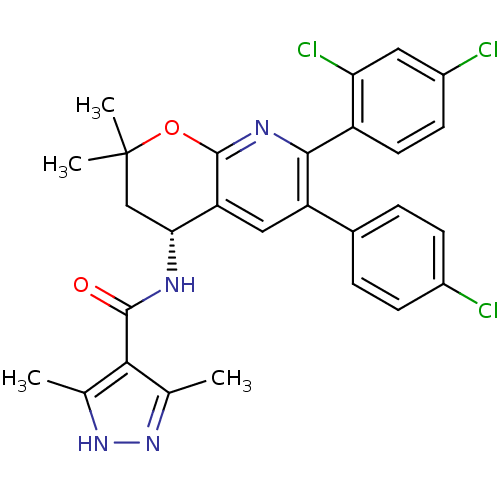

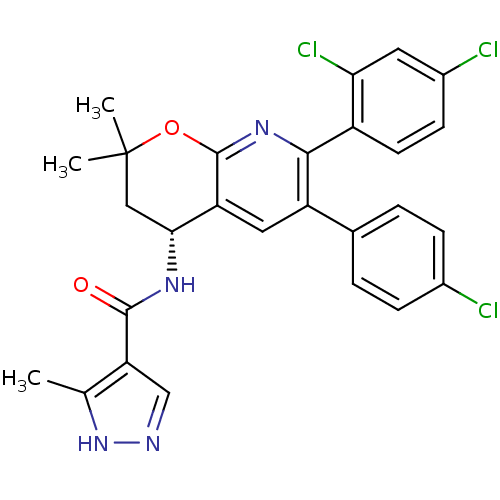
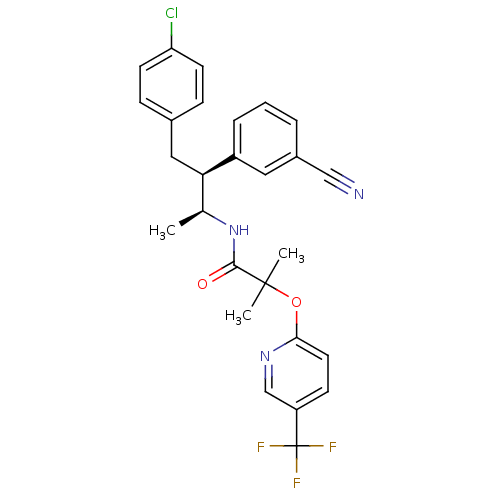
 3D Structure (crystal)
3D Structure (crystal)