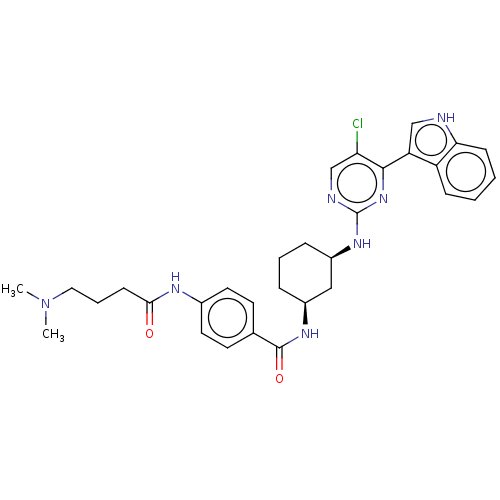
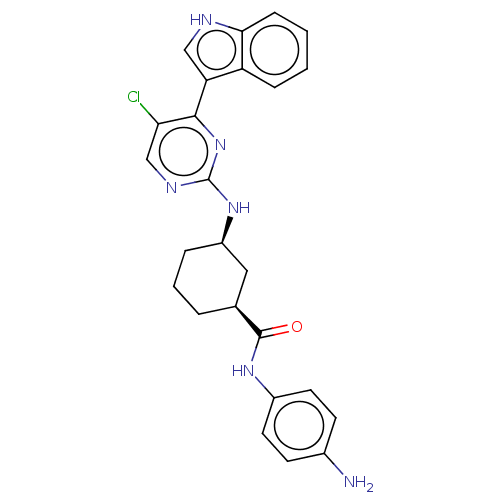
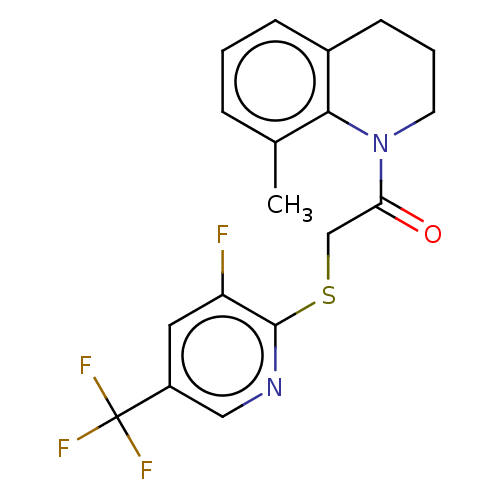
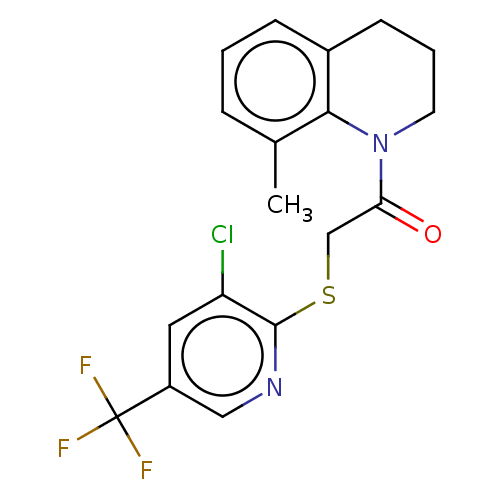
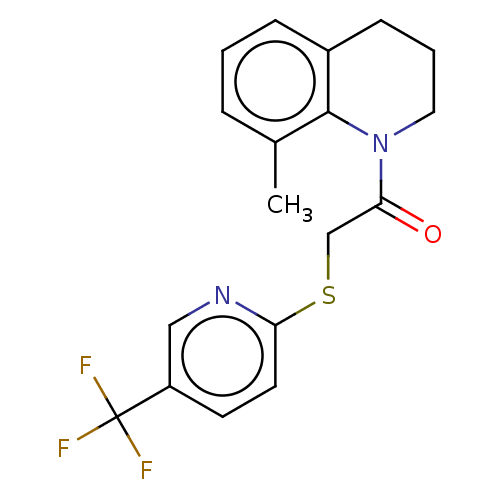
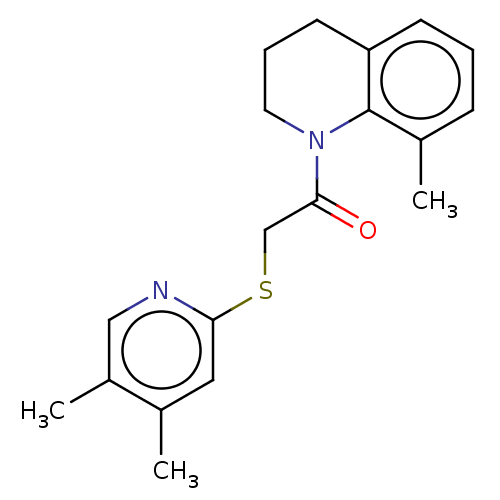
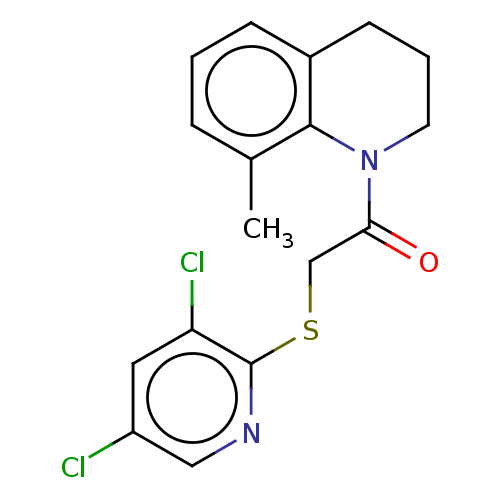
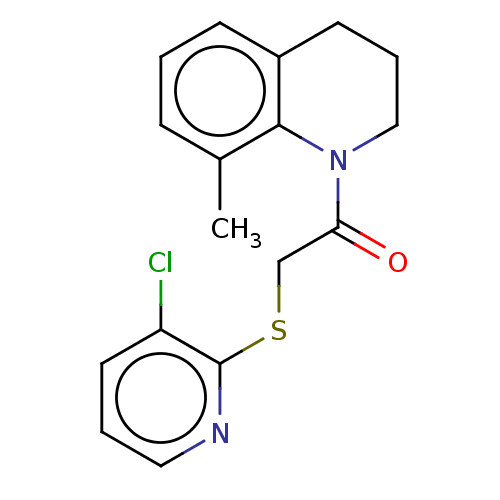
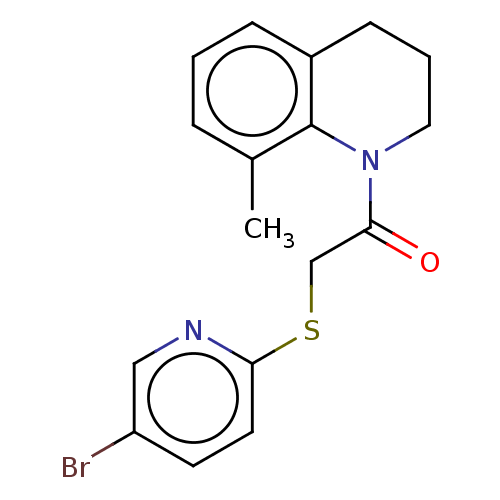
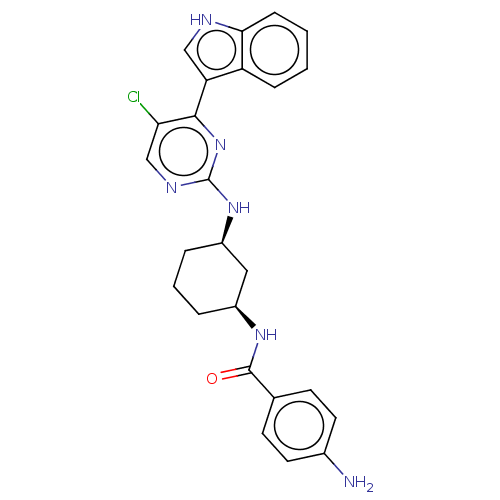
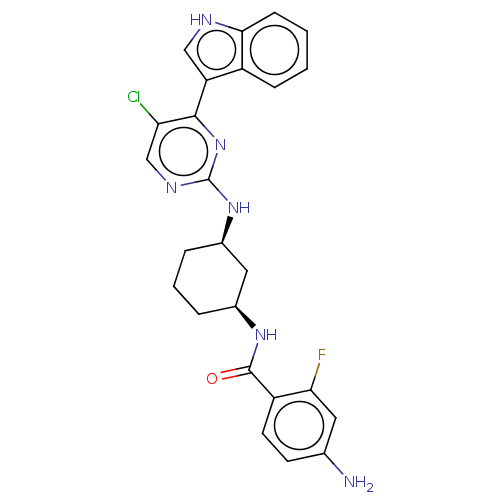
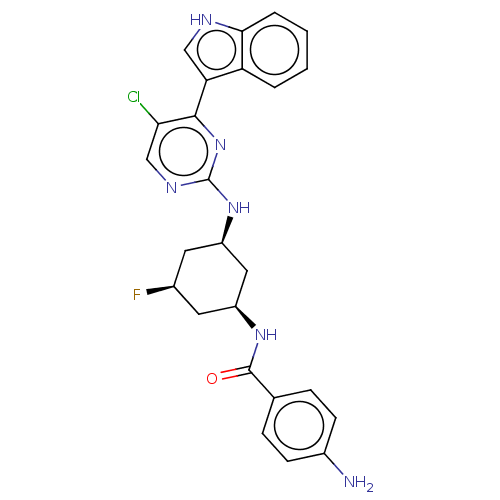
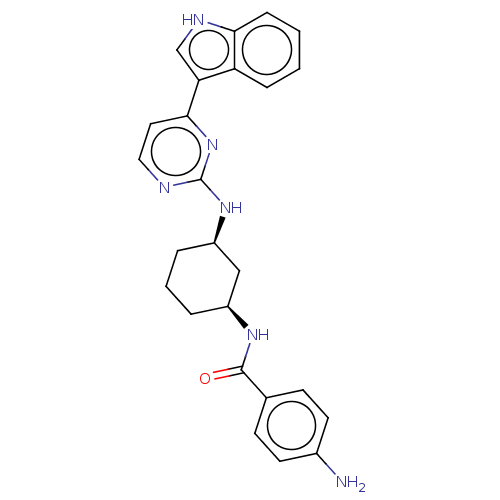
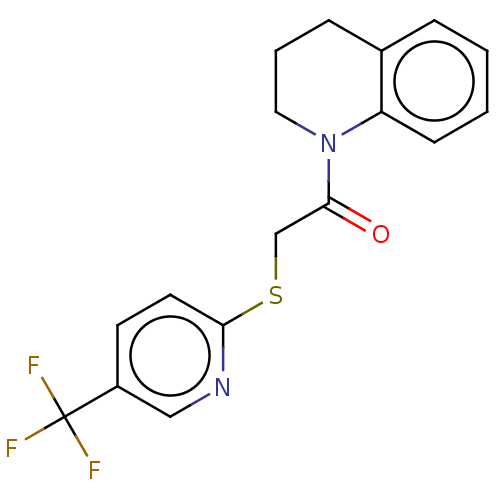
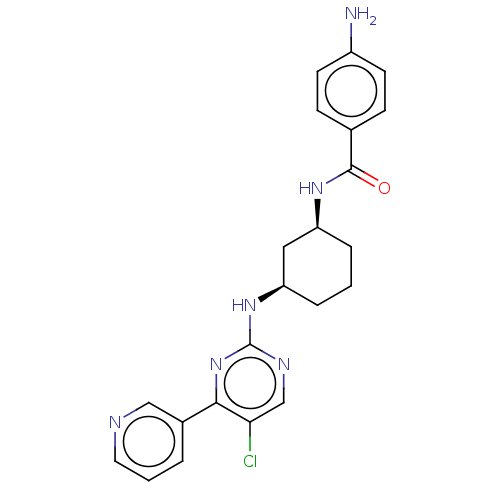
TargetRhizopuspepsin(Rhizopus microsporus var. chinensis)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Compound was evaluated for potency towards nicotinic acetylcholine receptor in rat P2 brain membranes using [3H]-nicotine as a radioligandMore data for this Ligand-Target Pair
TargetRhizopuspepsin(Rhizopus microsporus var. chinensis)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 336nMAssay Description:Compound was evaluated for inhibitory activity towards nicotinic acetylcholine receptor in rat P2 brain membranes using [3H]-nicotine as a radioligan...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies (Grand Island, N.Y.) using their commercially available Adapta kinase ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <1.00E+3nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <1.00E+3nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Compounds of the invention were assayed for CDK7 activity at Life Technologies(Grand Island, N.Y.) using their commercially available AdaptaŽ kinase ...More data for this Ligand-Target Pair