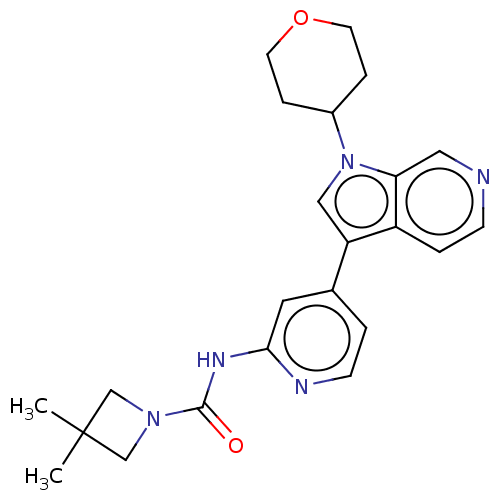
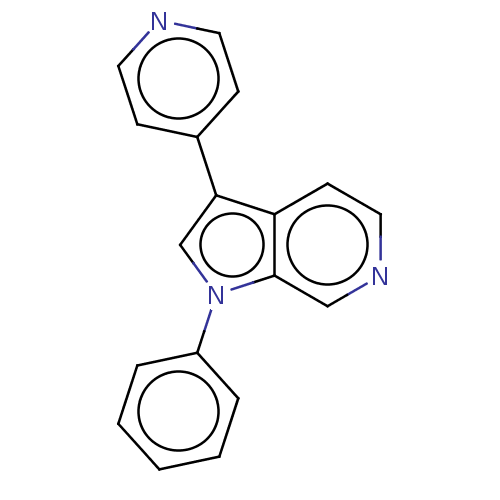
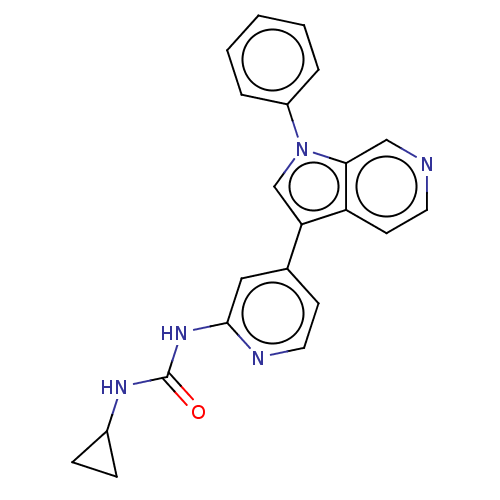
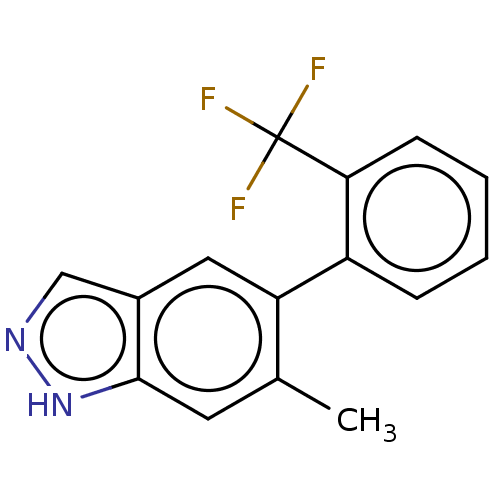
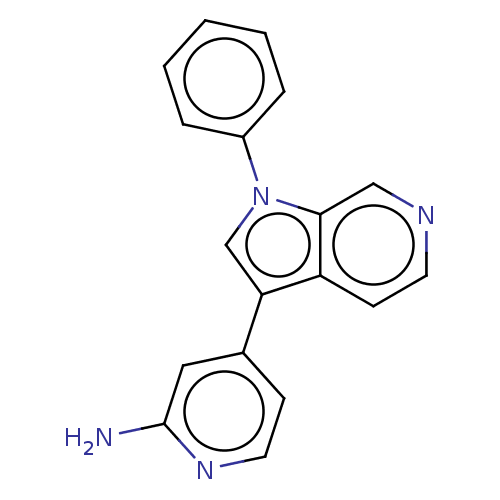
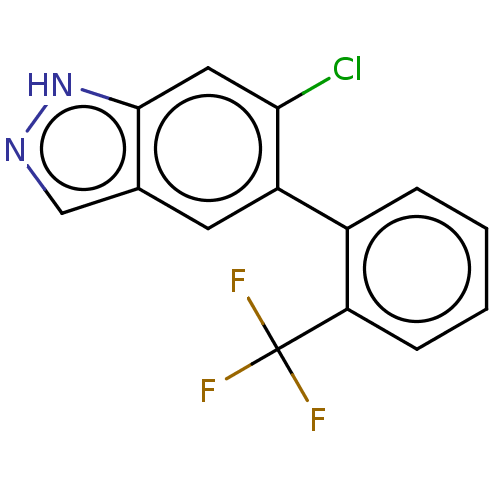
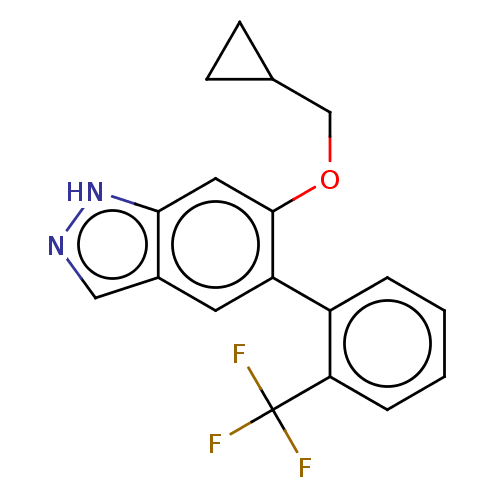
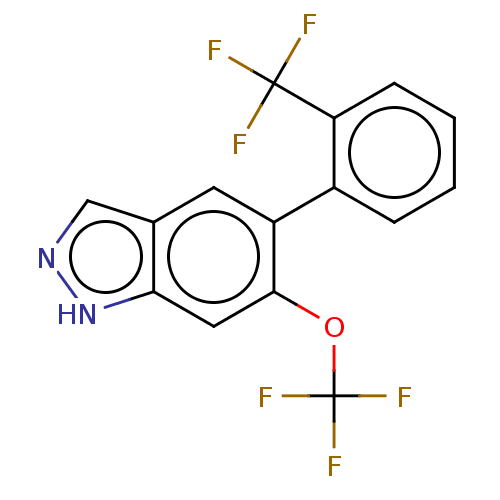
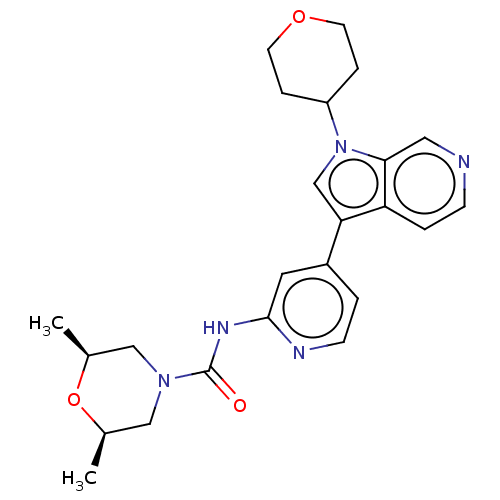
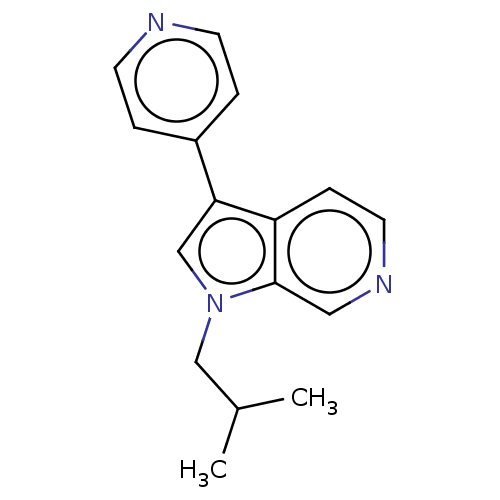
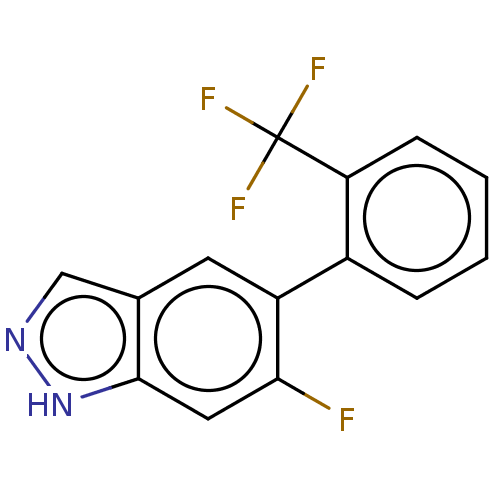
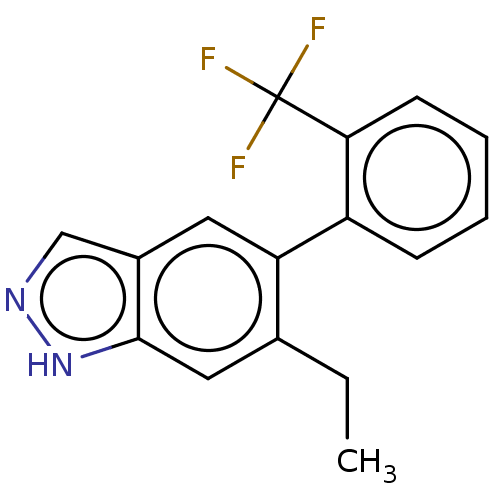
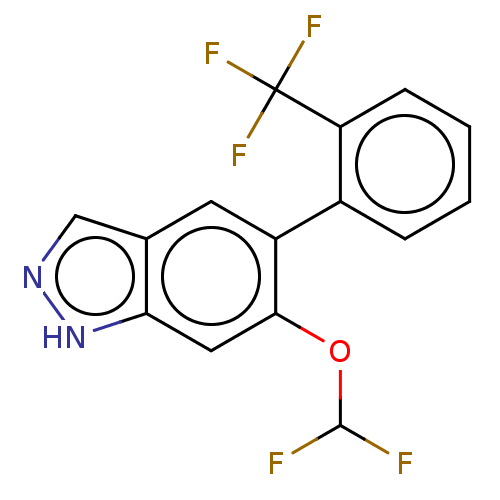
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 45nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 176nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 267nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.04E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.55E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.72E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.78E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.52E+4nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human DYRK1A (129 to 509 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of allyl isothiocyanate-induced calcium flux incubated 10 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of dibenzo[b,f ][1,4]oxazepine-4-carboxamide-induced calcium fl...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation (Gnf)
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity at human TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Mus musculus)
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Antagonist activity at mouse TRPA1 expressed in CHO-TREX cells assessed as inhibition of cinnamaldehyde-induced calcium flux incubated 10 mins prior ...More data for this Ligand-Target Pair

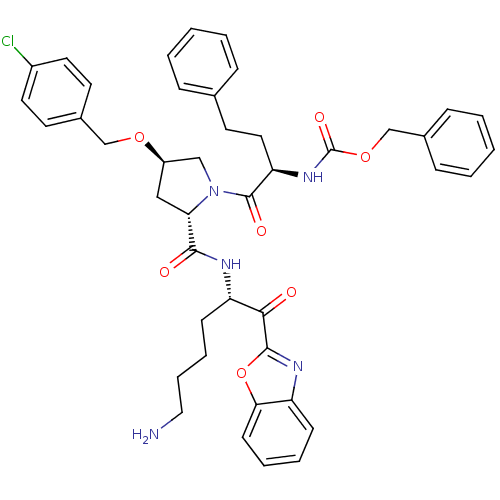
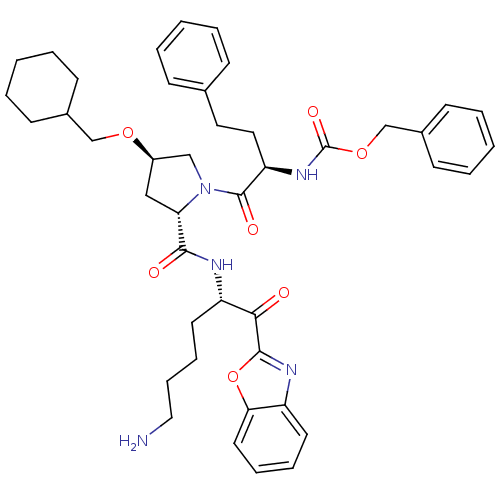
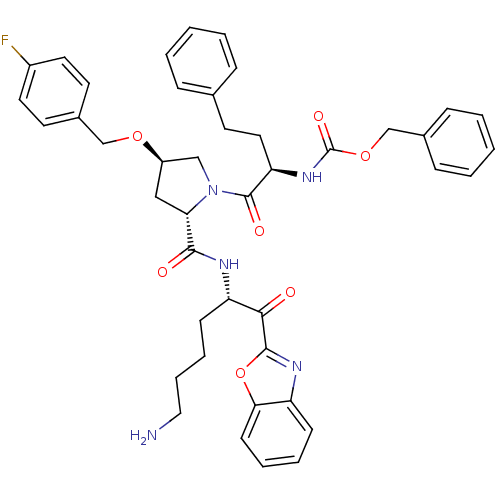

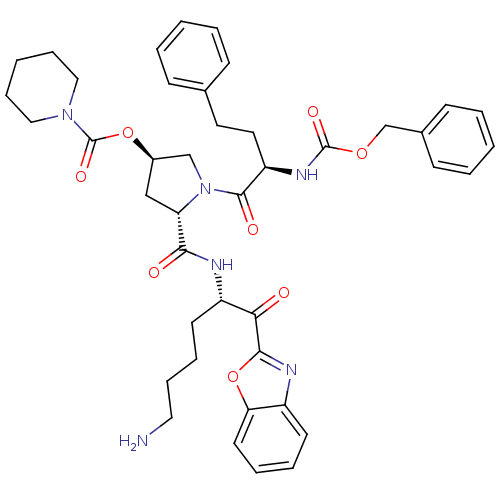
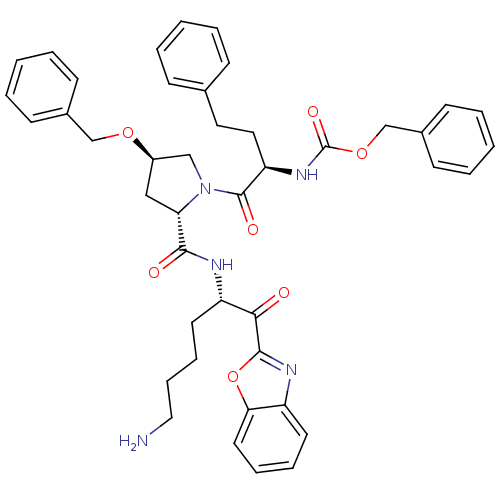
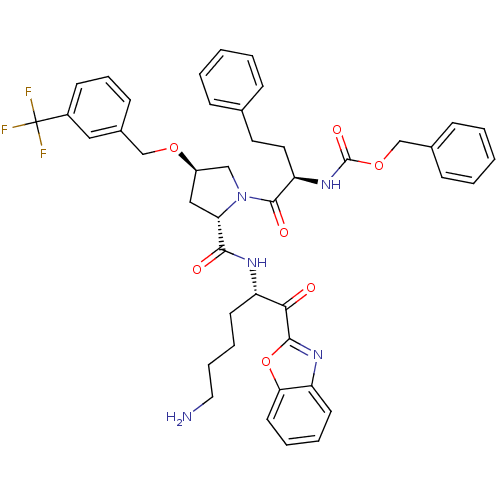
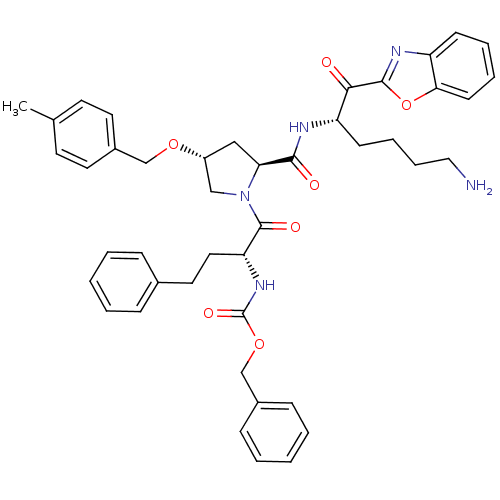

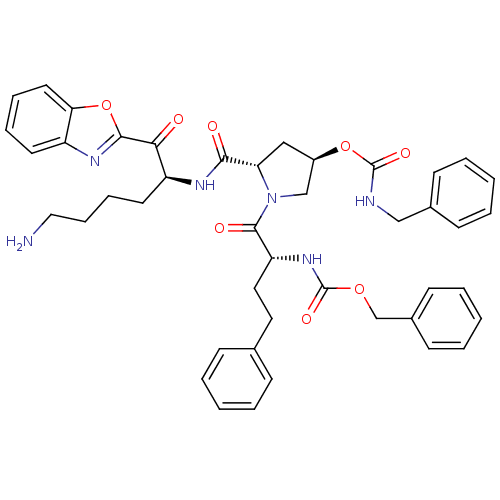



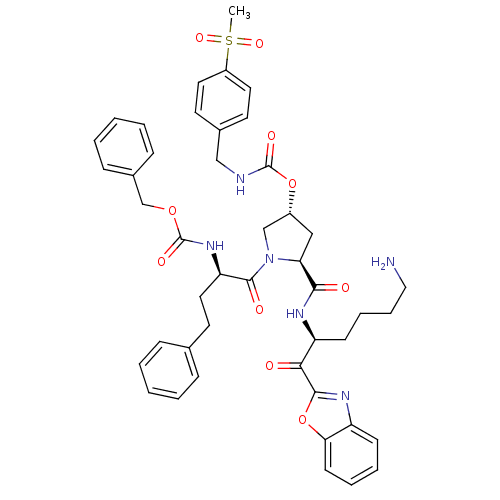
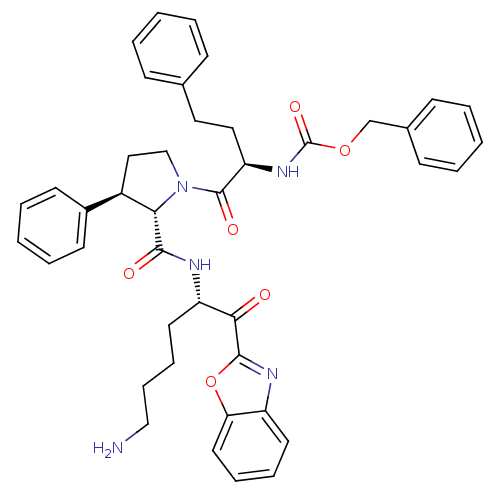
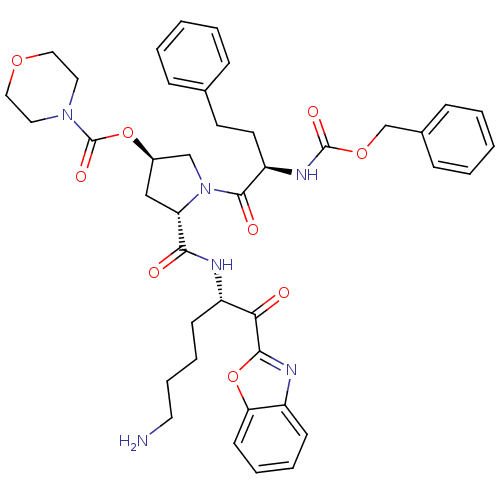



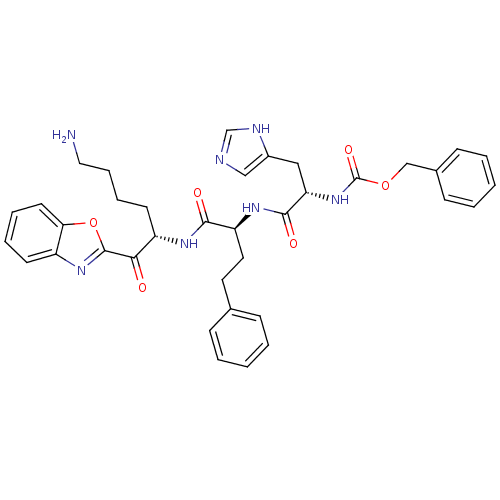
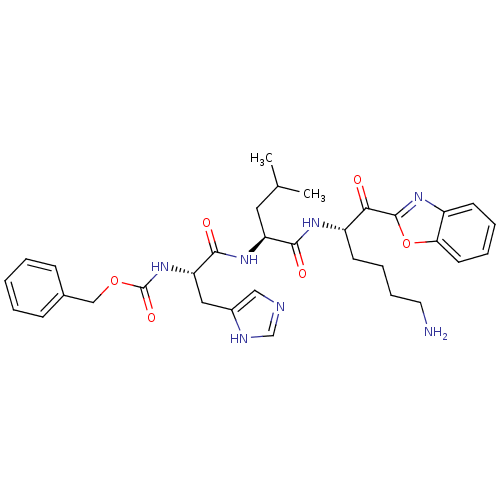
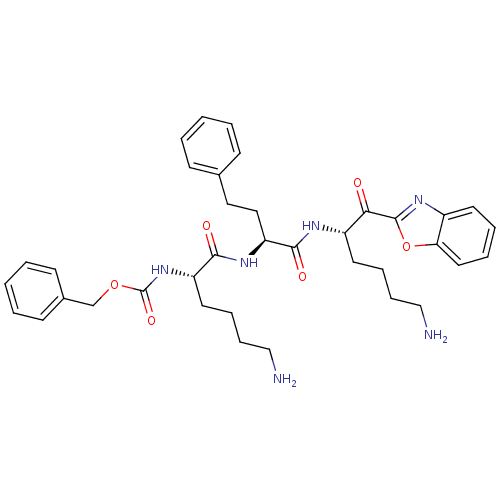


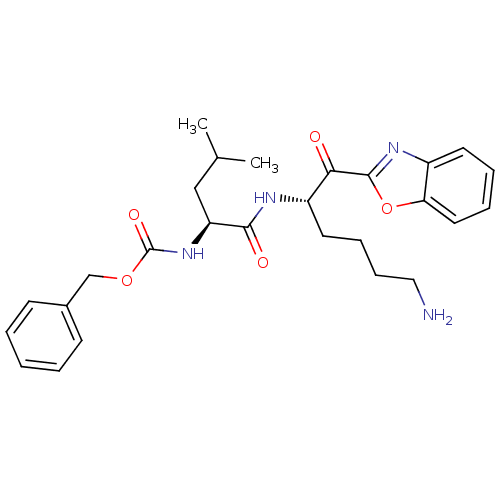



 3D Structure (crystal)
3D Structure (crystal)