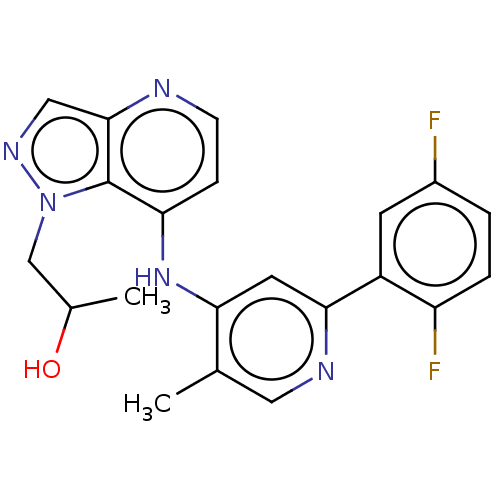
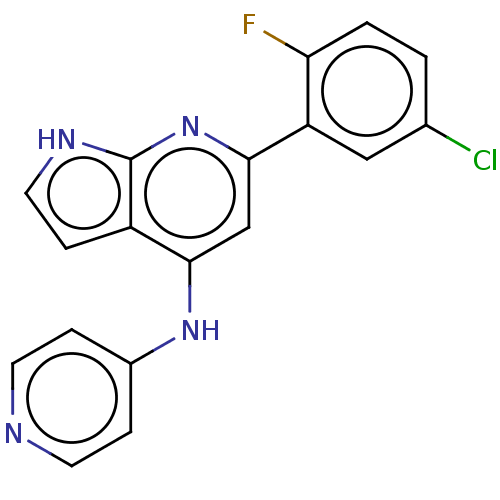
Affinity DataIC50: 0nMAssay Description:Inhibition of human Jak3More data for this Ligand-Target Pair
Affinity DataIC50: 0nMAssay Description:Inhibition of human EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 0nMAssay Description:Inhibition of human ITKMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: >2nMAssay Description:Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrateMore data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of MEK1 by IMAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: <3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of MEK1 by IMAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of MEK1 by IMAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of ALK5 (unknown origin) using fluorescein-labeled peptide substrate incubated for 90 mins by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of ALK5 (unknown origin) using fluorescein-labeled peptide substrate incubated for 90 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of MEK1 by IMAP assayMore data for this Ligand-Target Pair



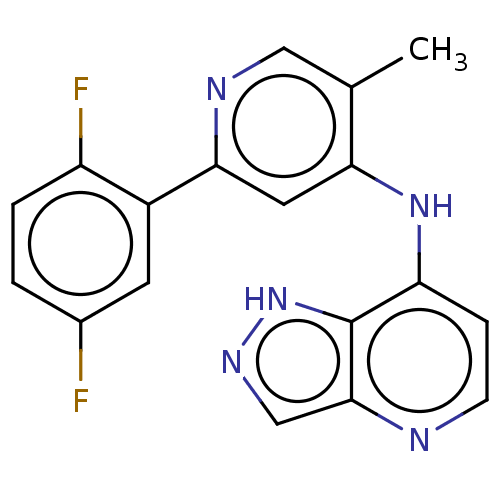
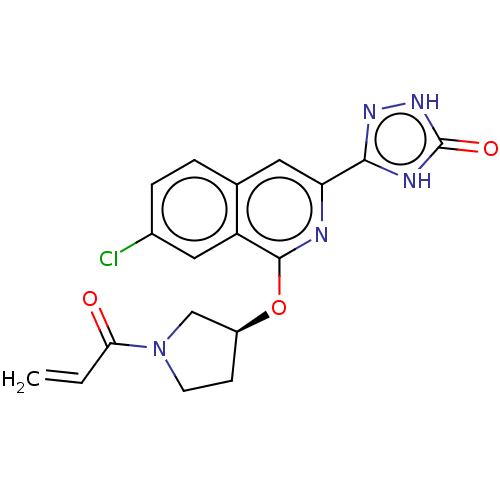
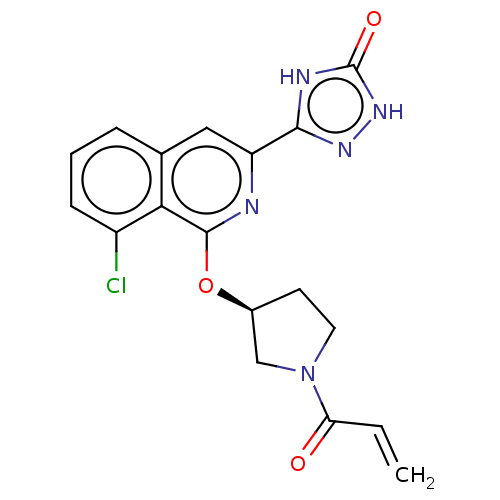
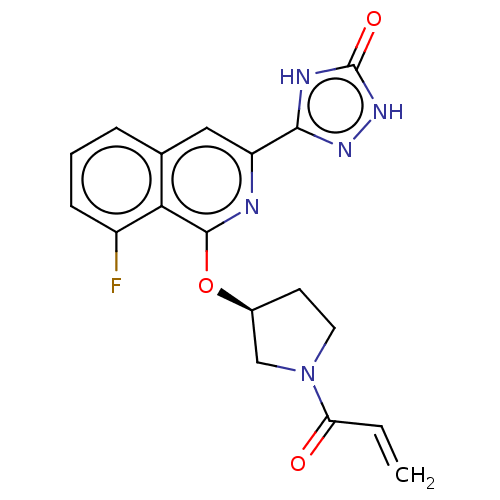
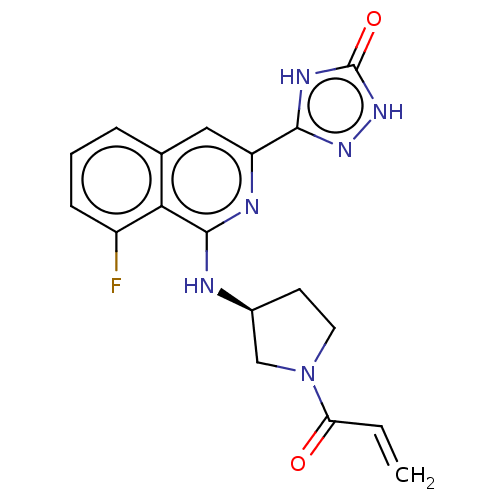
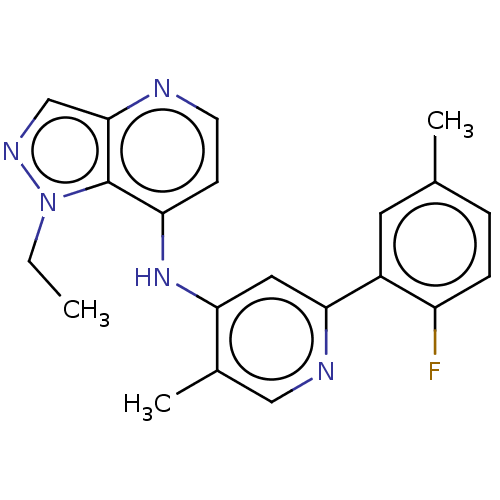


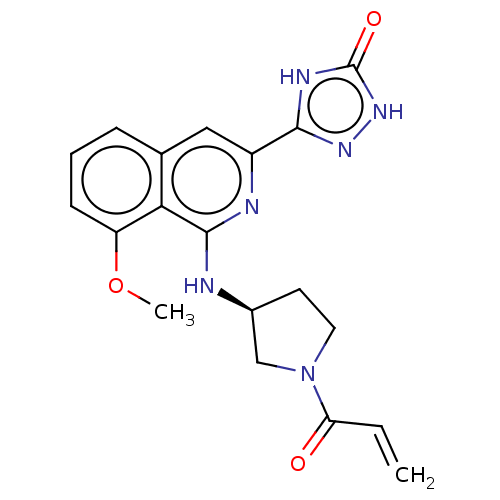
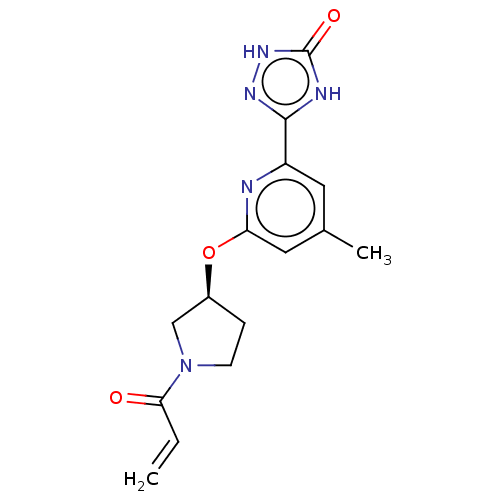
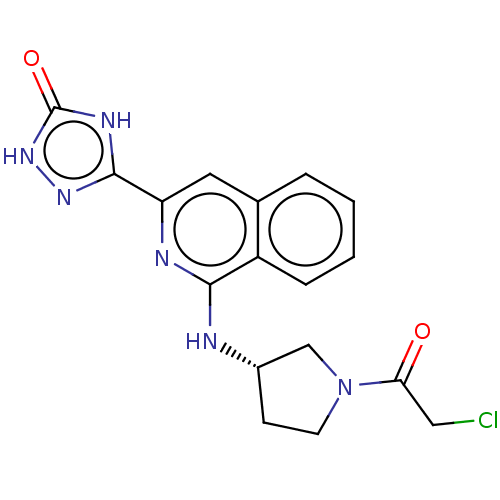
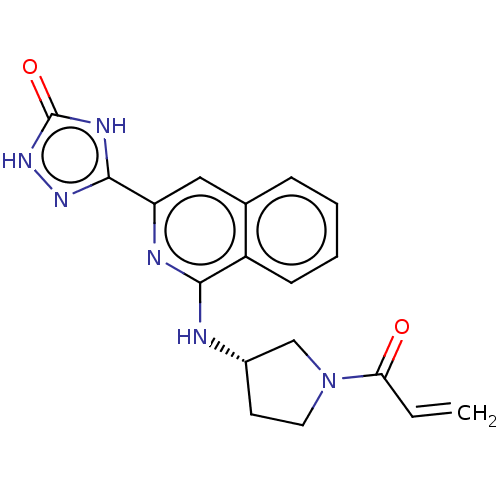
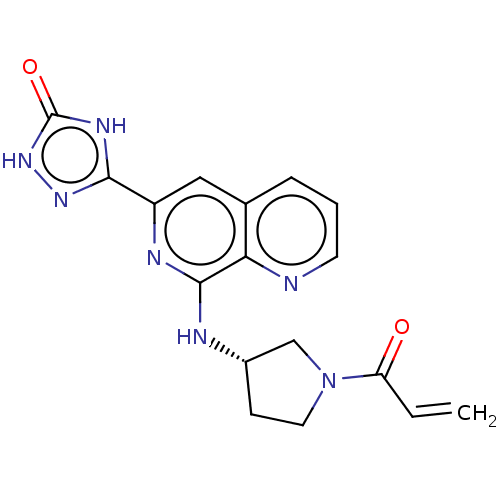
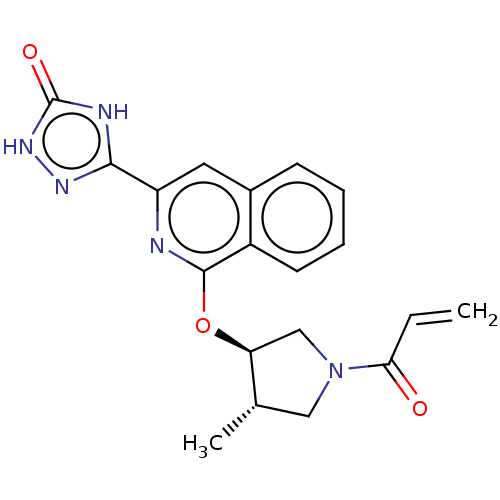
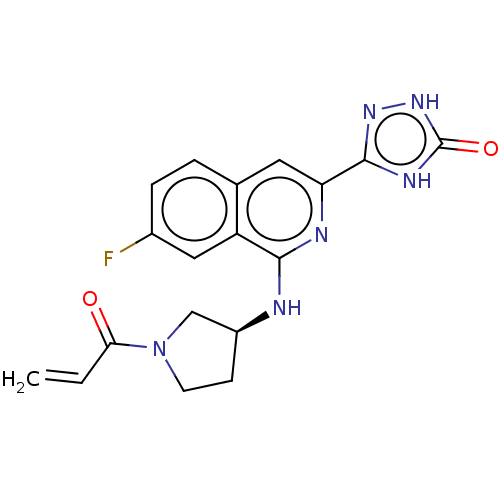
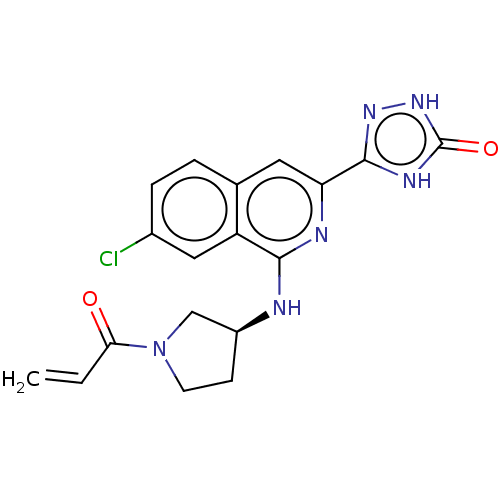
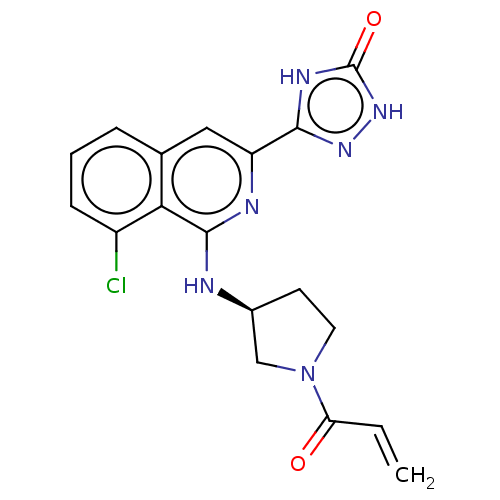

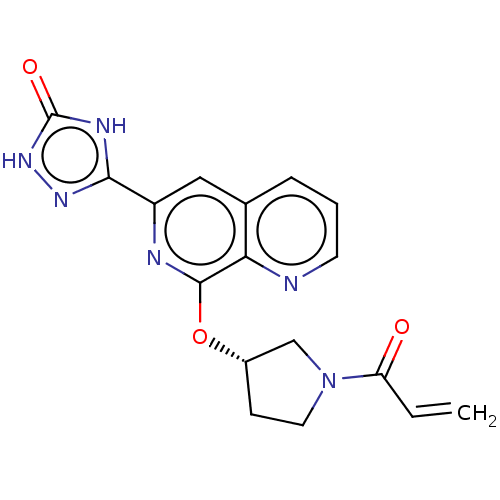
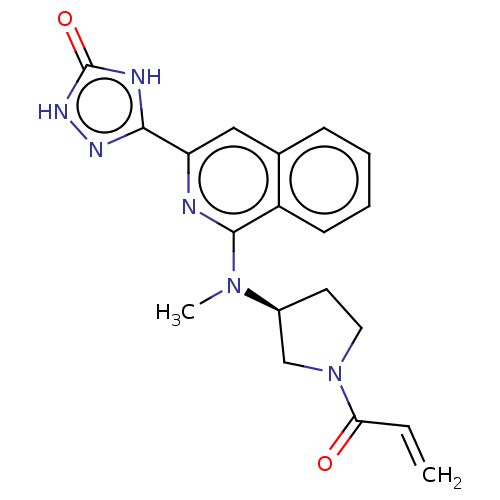
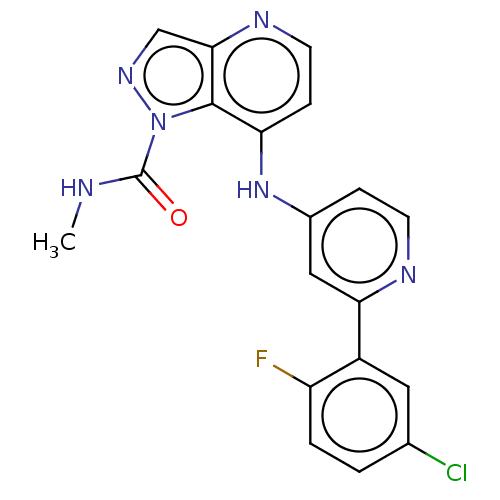

 3D Structure (crystal)
3D Structure (crystal)