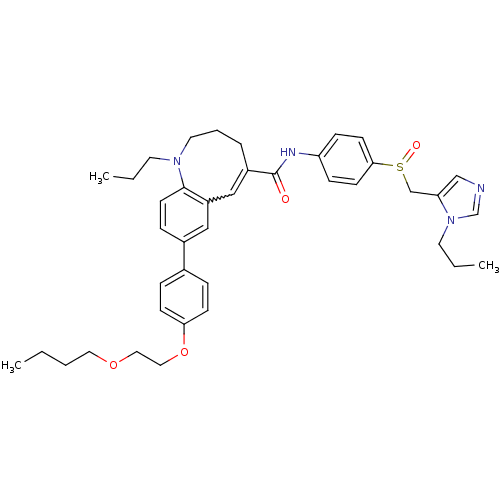
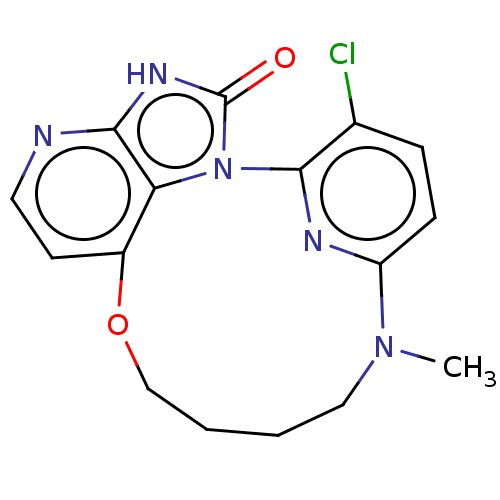
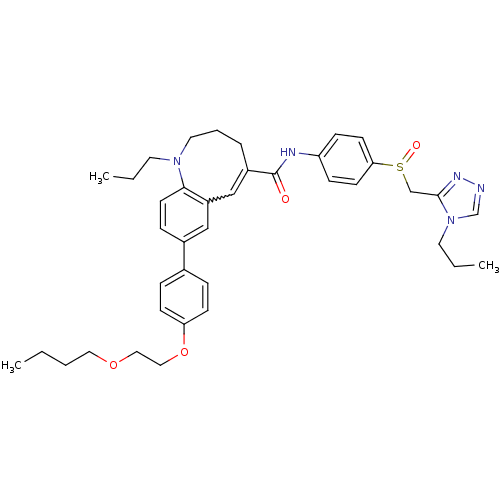
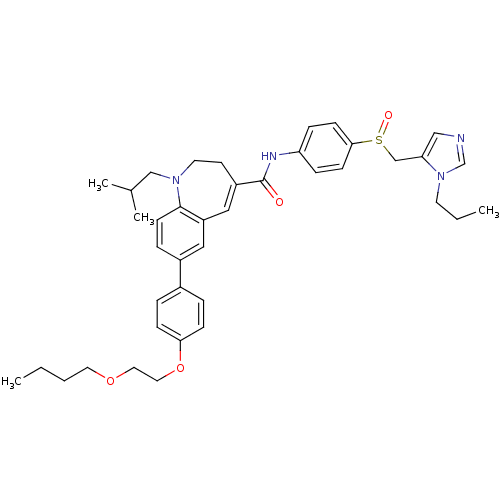
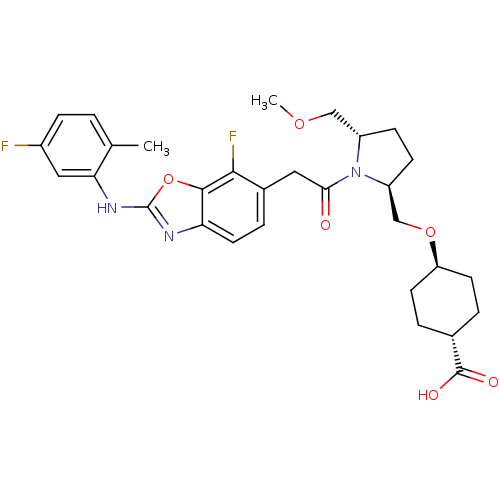
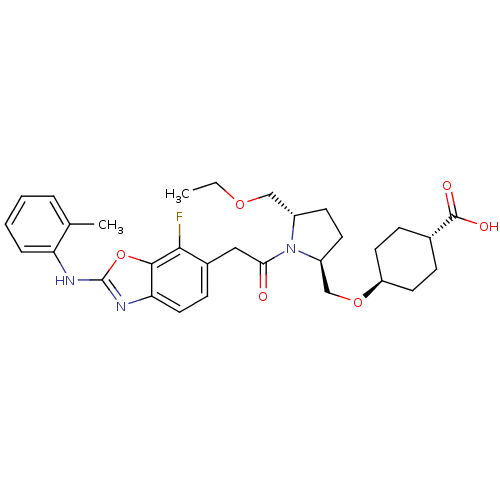
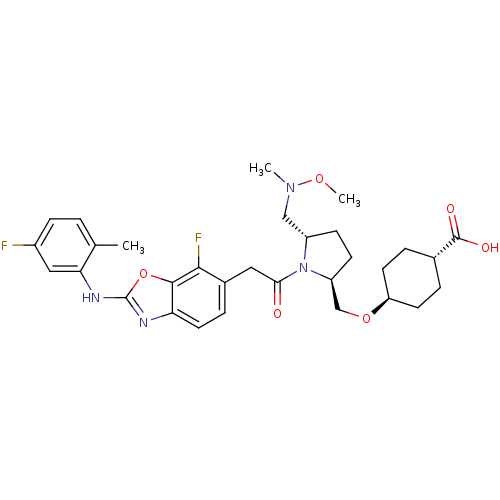
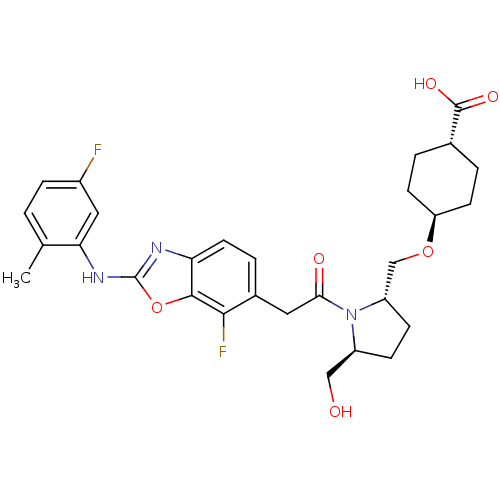
Affinity DataKi: 0.110nM ΔG°: -56.3kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
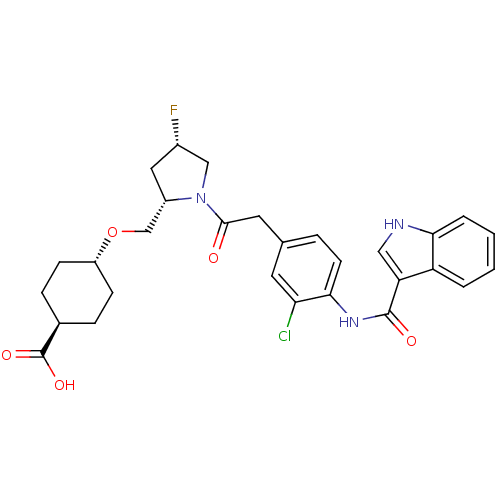
Affinity DataKi: 0.270nM ΔG°: -54.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
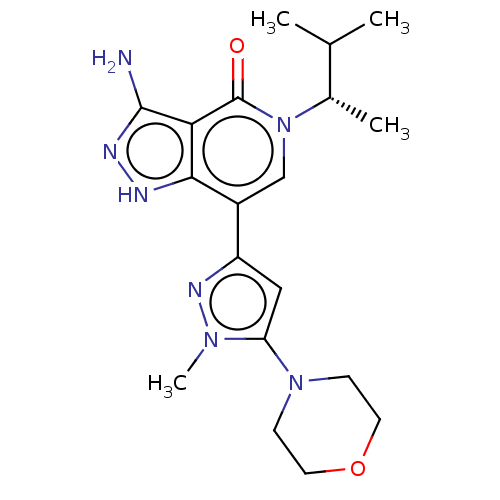
Affinity DataKi: 0.660nM ΔG°: -51.9kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
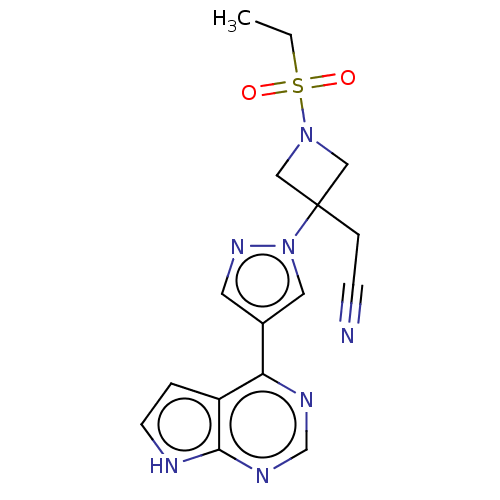
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -44.4kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -43.8kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.5kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 33nM ΔG°: -42.3kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 85nM ΔG°: -40.0kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 200nM ΔG°: -37.9kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 650nMAssay Description:Displacement of [3H] substance P from recombinant human NK1 receptor expressed in CHO cells after 90 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 870nM ΔG°: -34.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nM ΔG°: -33.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0860nMAssay Description:Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub...More data for this Ligand-Target Pair
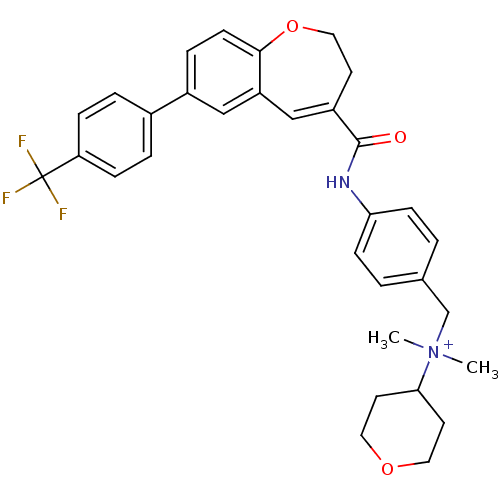
Affinity DataIC50: 0.100nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
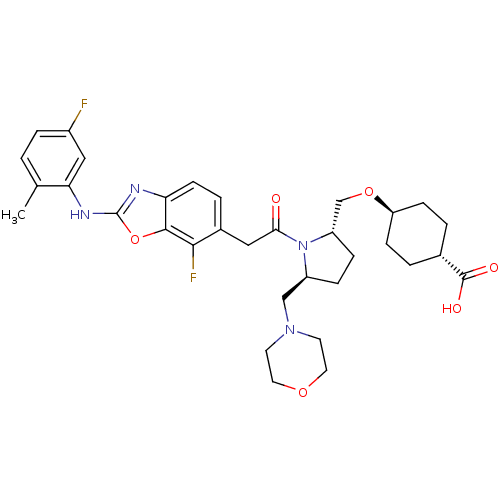
Affinity DataIC50: 0.510nMAssay Description:Inhibition of europium labeled human VCAM1/Fc chimera binding to human VLA-4 alpha-4-beta-1 expressed in Chinese hamster 4B4 cells by fluorimetric as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:Displacement of europium labeled human VCAM1 from human VLA4 expressed in 4B4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Inhibition of europium labeled human VCAM1/Fc chimera binding to human VLA-4 alpha-4-beta-1 expressed in Chinese hamster 4B4 cells by fluorimetric as...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
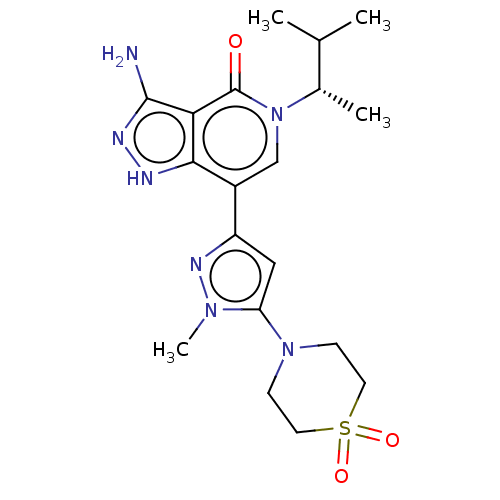
Affinity DataIC50: 0.550nMAssay Description:Inhibition of recombinant human TYK2 kinase domain (885-1176 residues) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent sub...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of JAK2 (unknown origin) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent substrate phosphorylation after 45 min...More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 0.920nMAssay Description:Inhibition of recombinant human TYK2 kinase domain (885-1176 residues) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent sub...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 0.980nMAssay Description:Inhibition of recombinant human TYK2 kinase domain (885-1176 residues) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent sub...More data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:Inhibition of JAK1 (unknown origin) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent substrate phosphorylation after 45 min...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Europium-labeled human VCAM1 binding to human VLA4 expressed in Chinese hamster 4B4 cells incubated for 60 mins by fluorometric assayMore data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of recombinant human TYK2 kinase domain (885-1176 residues) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent sub...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Europium-labeled human VCAM1 binding to human VLA4 expressed in Chinese hamster 4B4 cells incubated for 60 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
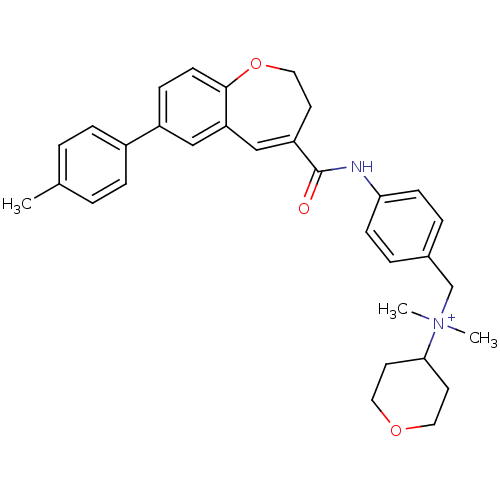
Affinity DataIC50: 1.40nMAssay Description:Displacement of [125I]RANTES from CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTESMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTESMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTESMore data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of recombinant human TYK2 kinase domain (885-1176 residues) using Ulight-JAK1 substrate peptide assessed as reduction in ATP-dependent sub...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Displacement of europium labeled human VCAM1 from human VLA4 expressed in 4B4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [125I]RANTES from CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of Europium-labeled human VCAM1 binding to human VLA4 expressed in Chinese hamster 4B4 cells incubated for 60 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of Europium-labeled human VCAM1 binding to human VLA4 expressed in Chinese hamster 4B4 cells incubated for 60 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of Europium-labeled human VCAM1 binding to human VLA4 expressed in Chinese hamster 4B4 cells incubated for 60 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [125I]RANTES from CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair




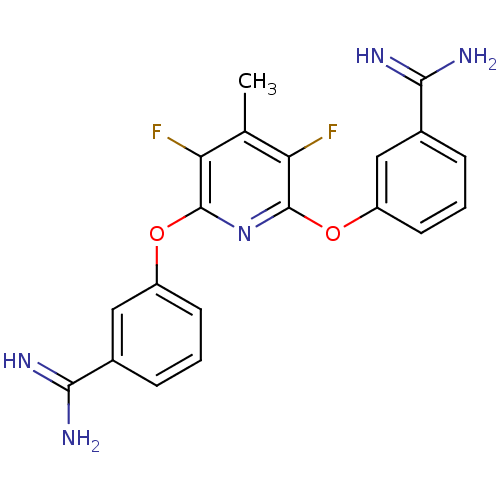
 3D Structure (crystal)
3D Structure (crystal)