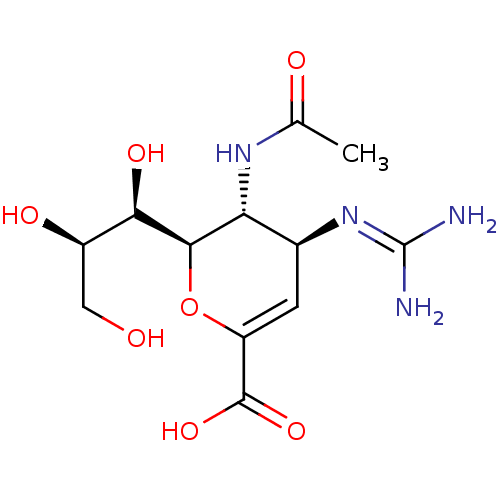
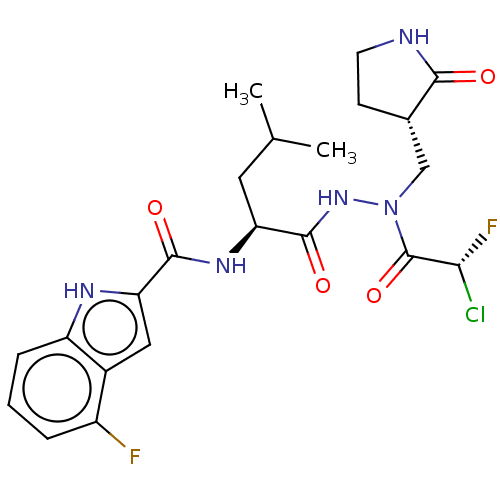
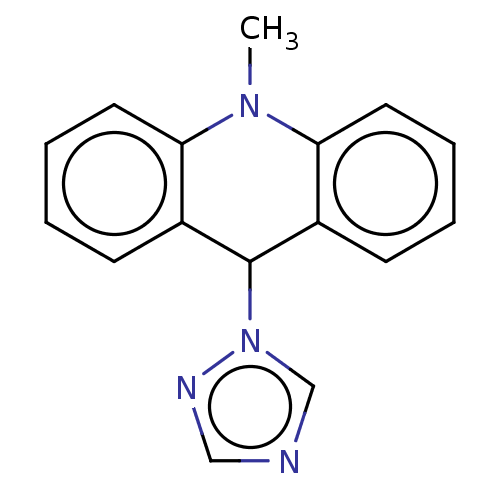
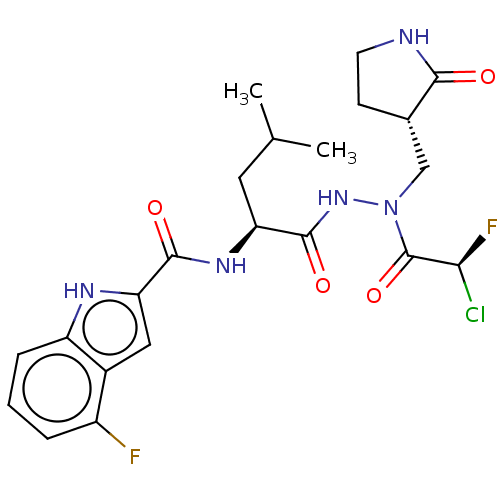
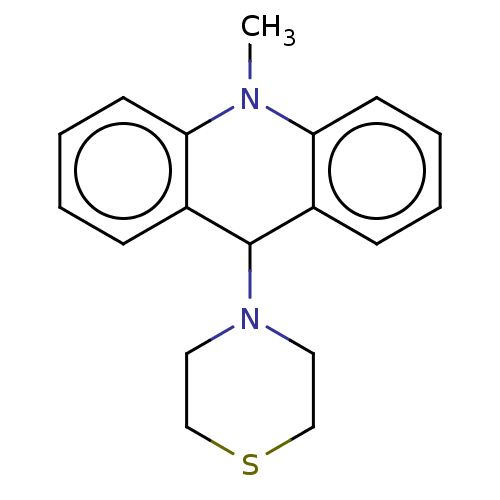
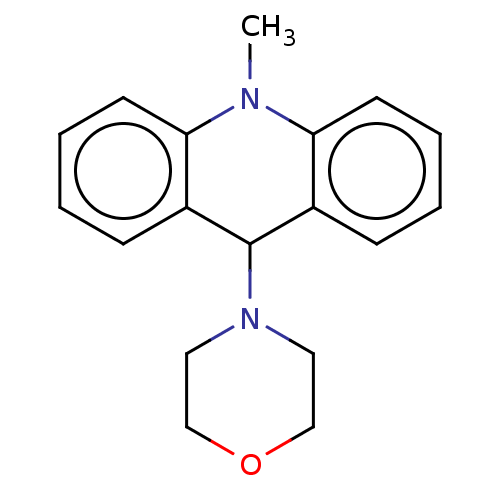
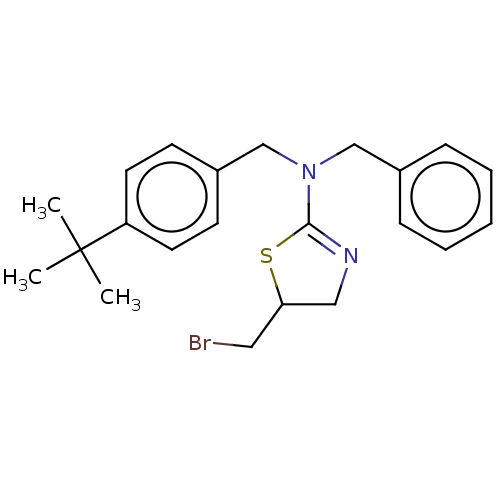
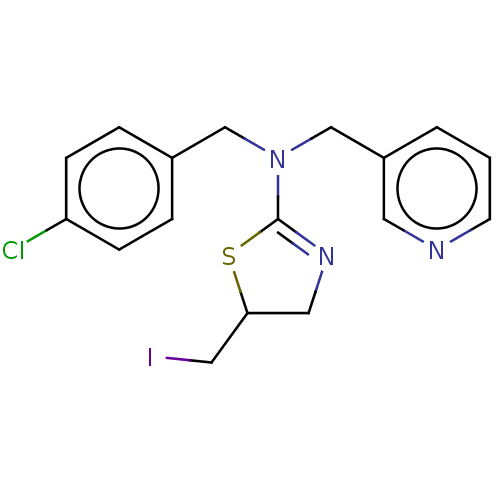
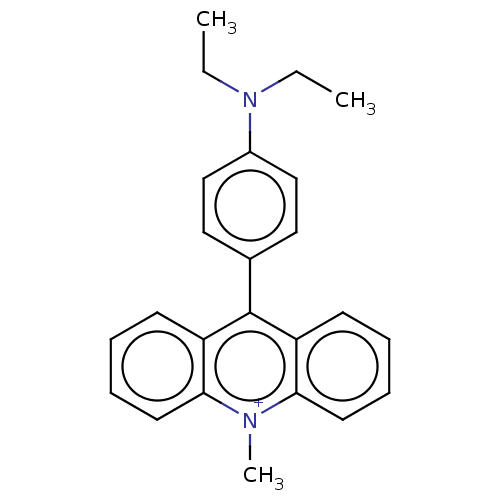
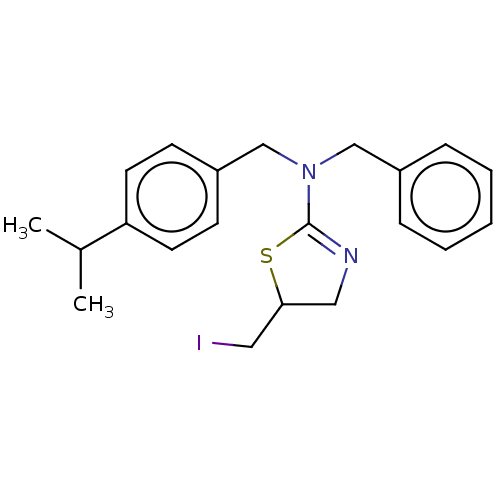
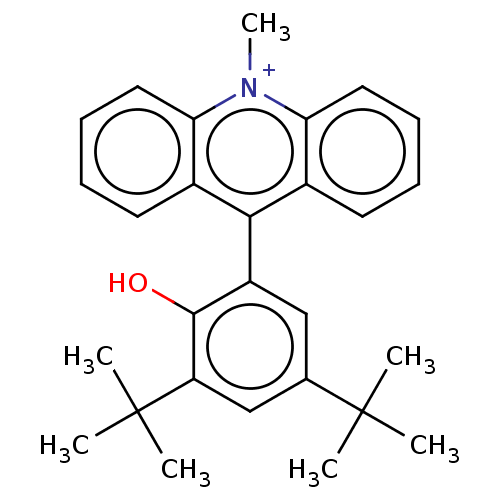
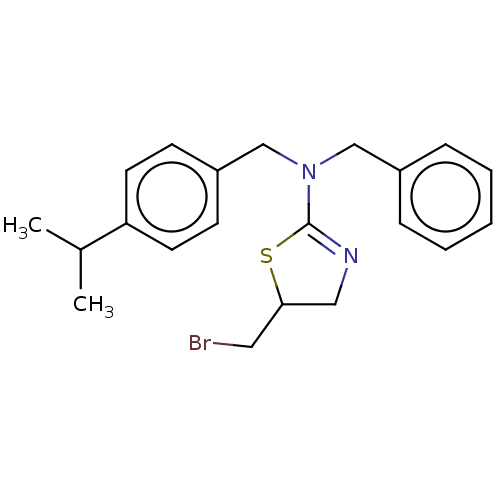
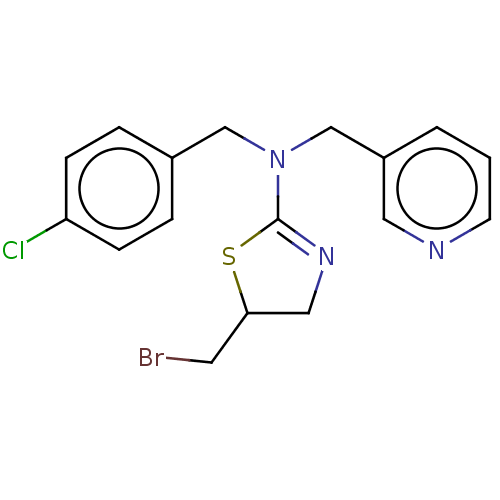
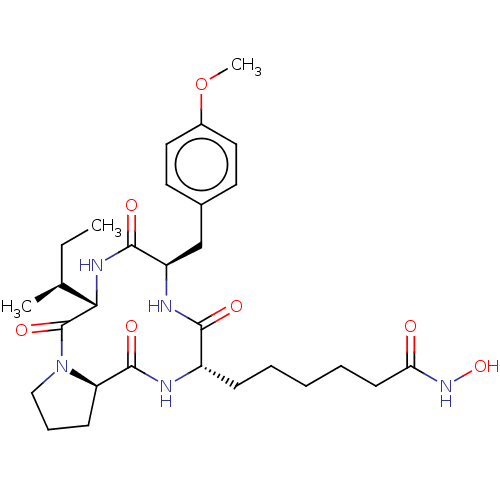
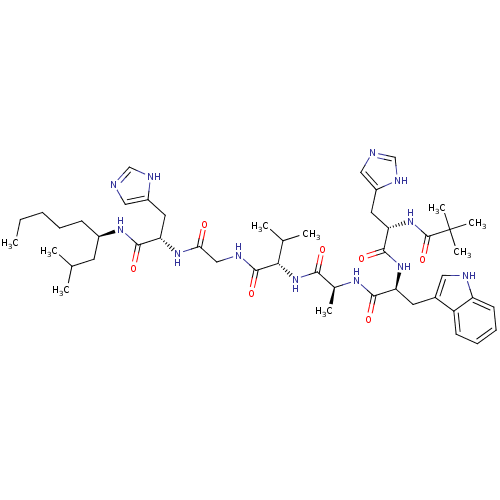
Affinity DataKi: 0.100nMAssay Description:Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.90nMAssay Description:Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Irreversible inhibition of recombinant full length SARS-CoV-2 3CLpro expressed in Escherichia coli using Ac-Abu-Tle-Leu-Gln-MCA as fluorogenic substr...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
Affinity DataKi: 224nMAssay Description:Irreversible inhibition of recombinant full length SARS-CoV-2 3CLpro expressed in Escherichia coli using Ac-Abu-Tle-Leu-Gln-MCA as fluorogenic substr...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 270nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 440nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 490nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 620nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 670nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 940nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.34E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.46E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.53E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 2.09E+3nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 2.66E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.34E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 3.54E+3nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.60E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.75E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 4.01E+3nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 6.43E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 7.37E+3nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 7.56E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 7.81E+3nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 9.51E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.32E+4nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of recombinant Set7/9 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Ac-KRSK-MCA peptide/SAM as substrate preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.99E+4nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human HDAC1 expressed in HEK293T cells assessed as aminomethyl coumarin release using Ac-KGLGK(Ac)-MCA) substrate after 30 mins by FLIP...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against histone deacetylases (HDAC1) prepared from mouse melanoma B16/BL6 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:The phosphorylation activity on the peptide substrate of EGFR was investigated using LabChip (trademark) Systems (Caliper Life Sciences, Inc.). For t...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Binding affinity to human recombinant BACE-1 (1 to 460 residue) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition...More data for this Ligand-Target Pair
TargetGastrin-releasing peptide receptor(MOUSE)
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition against Swiss 3T3 murine fibroblast cells.More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)