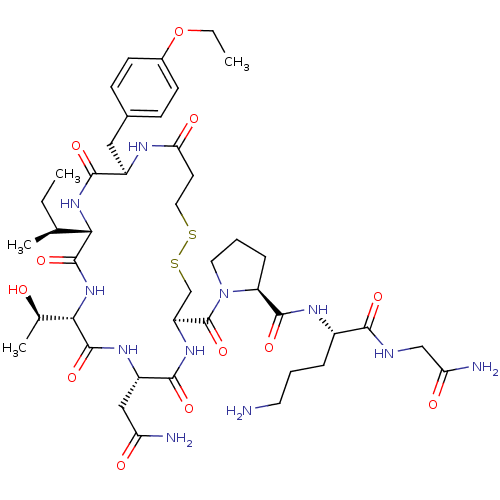
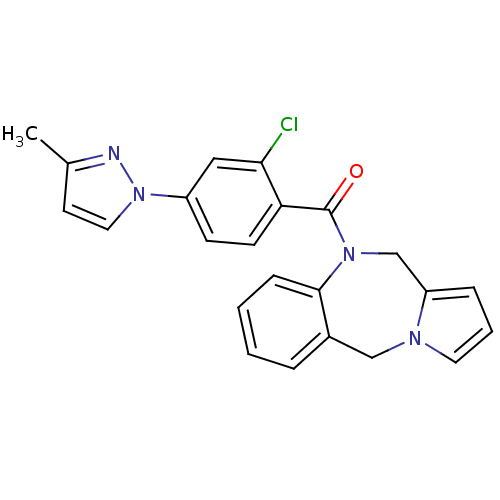
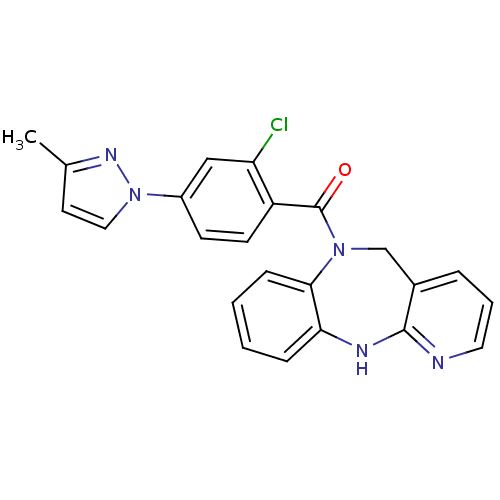
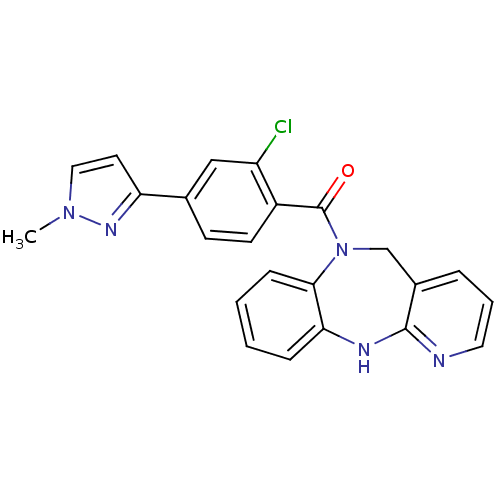
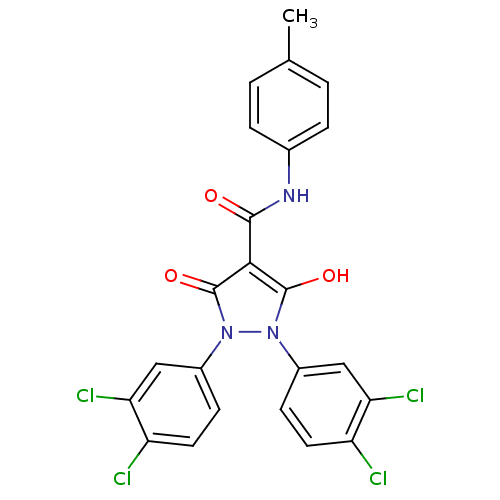
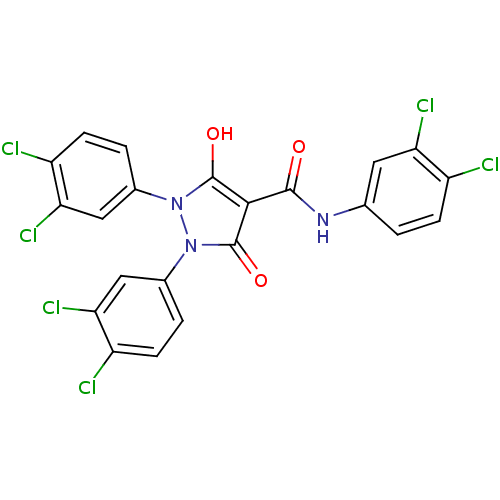
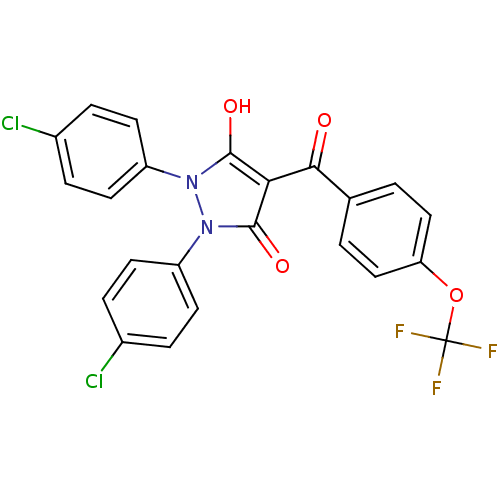
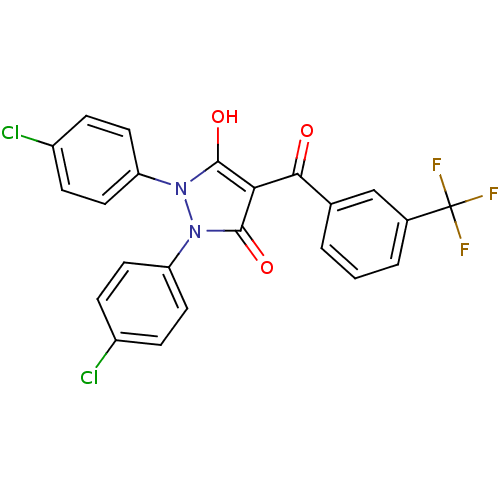
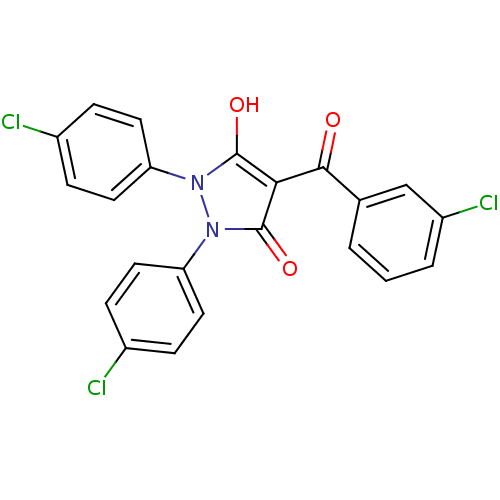
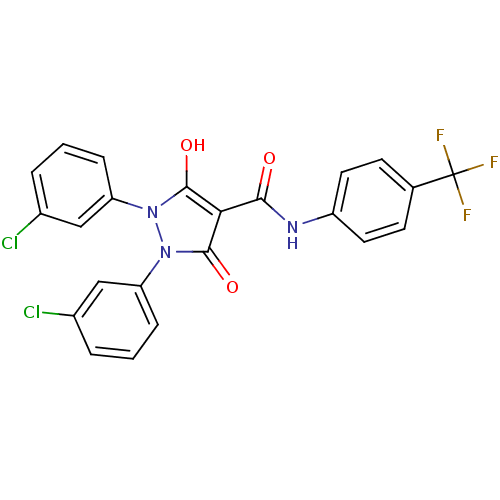
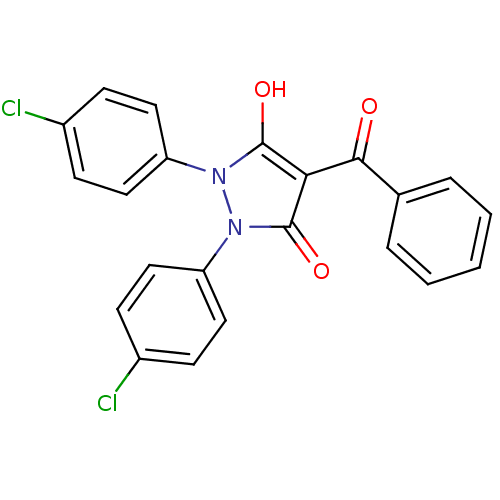
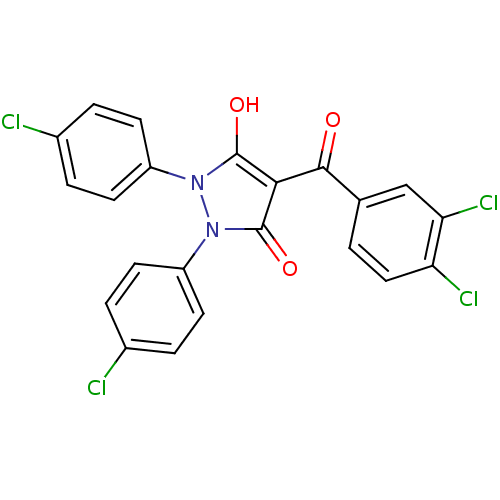
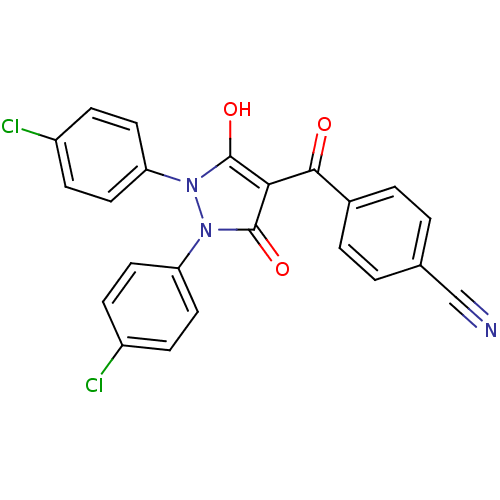
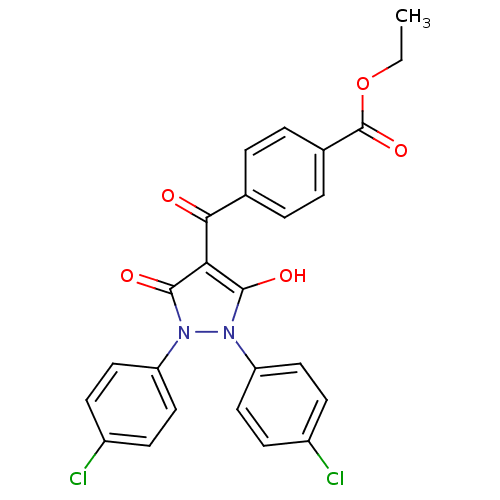
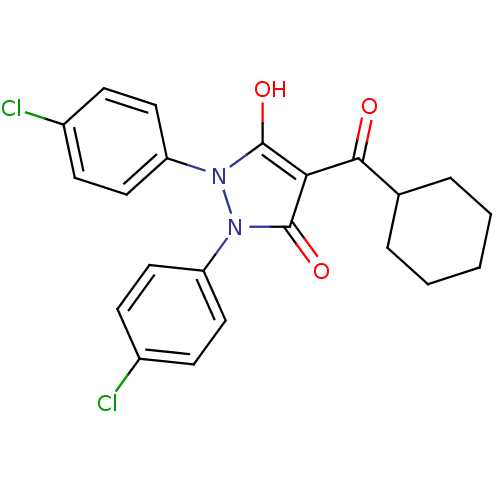
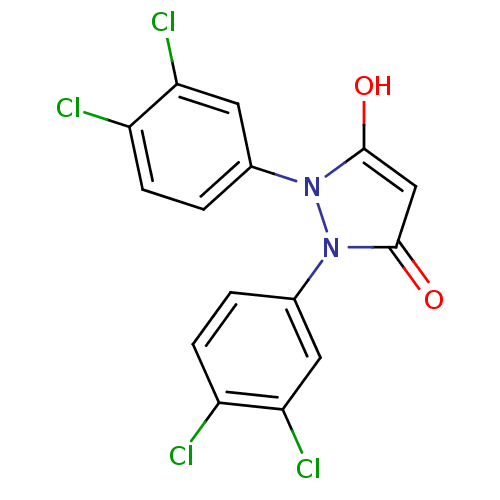
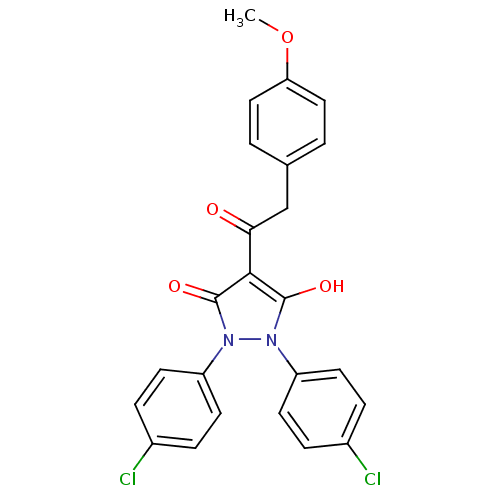
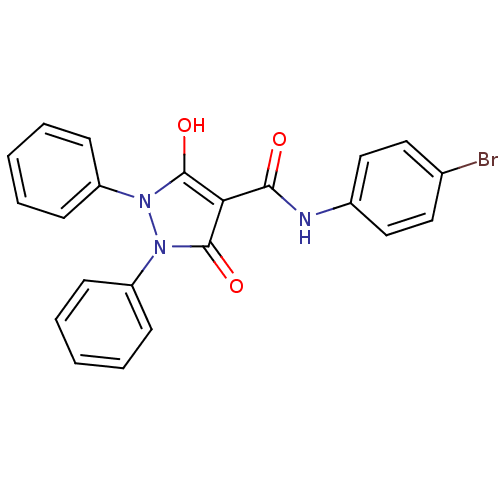
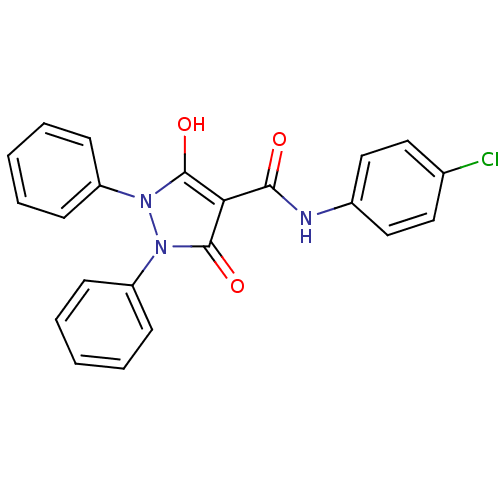
Affinity DataIC50: 12nMAssay Description:Antagonism of OT-induced response at OT receptor in rat uterine stripsMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 91.5nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 183nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 296nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 413nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 475nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 493nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 778nMAssay Description:Displacement of [3H]AVP from human V1a receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 933nMAssay Description:Displacement of [3H]AVP from human V2 receptor transfected in LV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Antagonism of OT-induced response at OT receptor in rat uterine stripsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+3nMAssay Description:Displacement of [3H]AVP from human V1a receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
Affinity DataIC50: 5.95E+3nMAssay Description:Displacement of [3H]AVP from human V1a receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 8.60E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 8.70E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.22E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.31E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 1.76E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 2.19E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 2.51E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Antagonism of AVP-induced response at V1a receptor in isolated rat tail arteriesMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: >4.40E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: >5.20E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: >5.50E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair
TargetUDP-N-acetylenolpyruvoylglucosamine reductase(Escherichia coli K-12 (Enterobacteria))
Wyeth
Curated by ChEMBL
Wyeth
Curated by ChEMBL
Affinity DataIC50: 6.85E+4nMAssay Description:Inhibition of MurB activityMore data for this Ligand-Target Pair