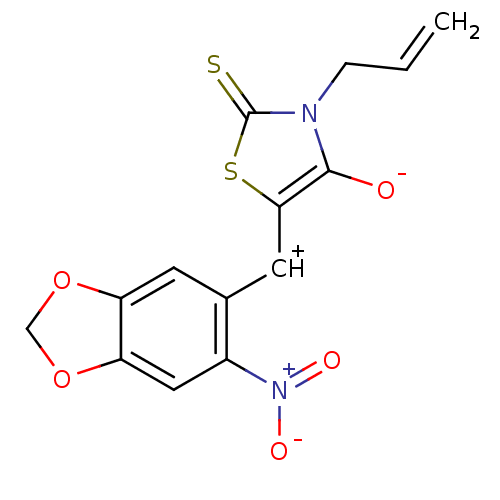
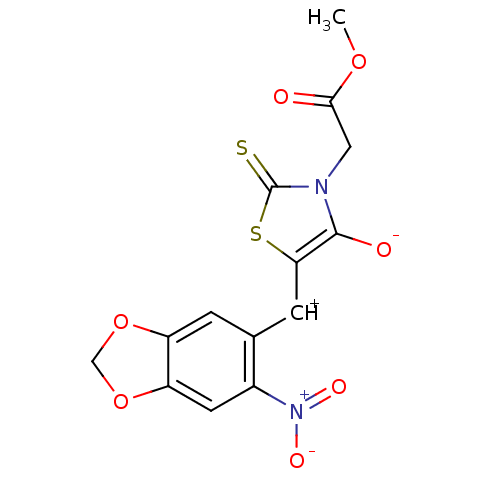
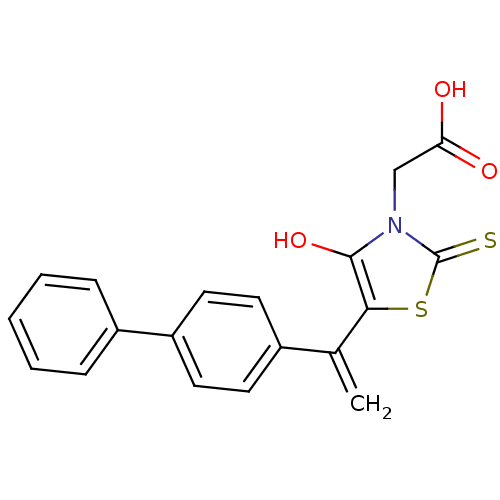
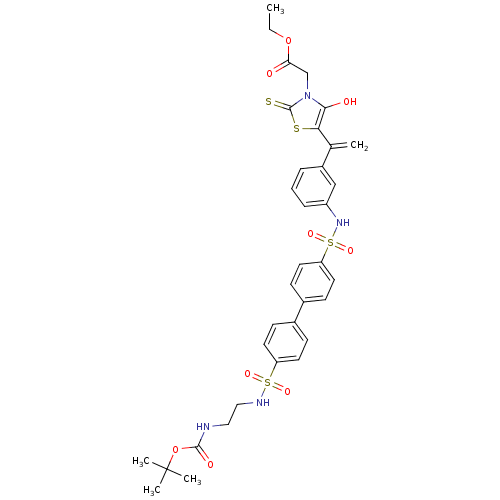
Affinity DataIC50: <1.00E+3nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity of the compound against plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+4nMAssay Description:Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+5nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 3.75E+5nMAssay Description:Inhibitory activity of the compound against Serine protease chymotrypsinMore data for this Ligand-Target Pair