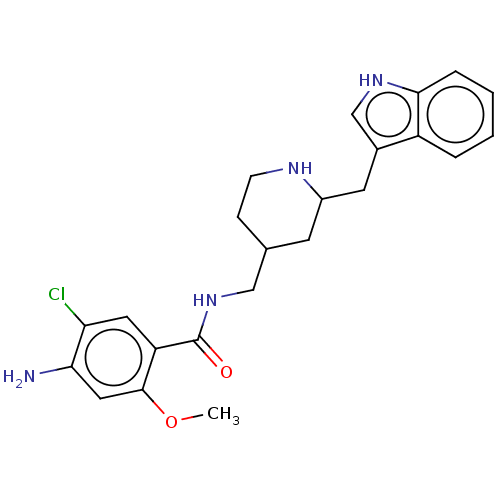
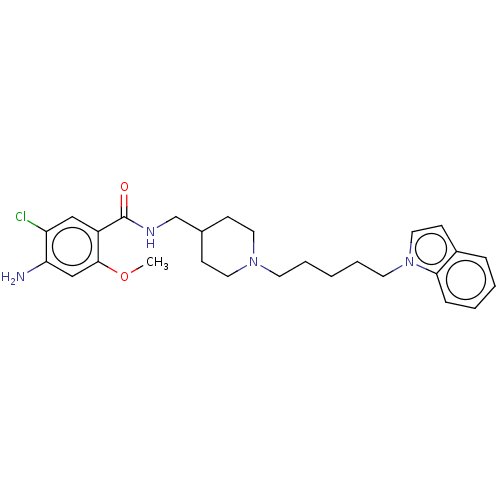
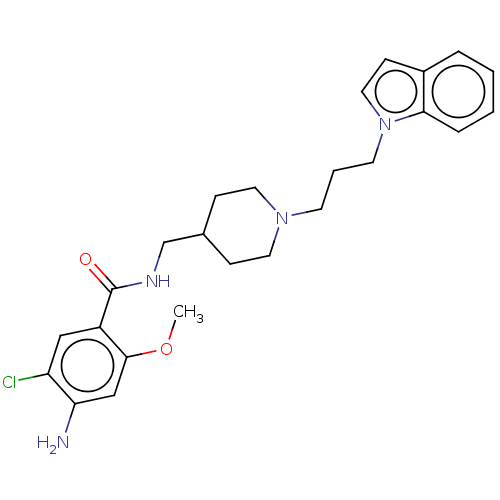
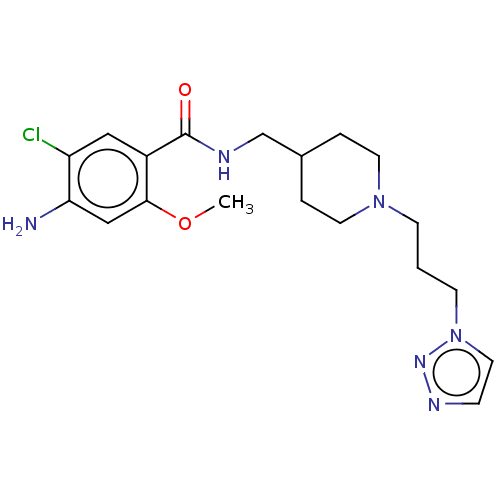
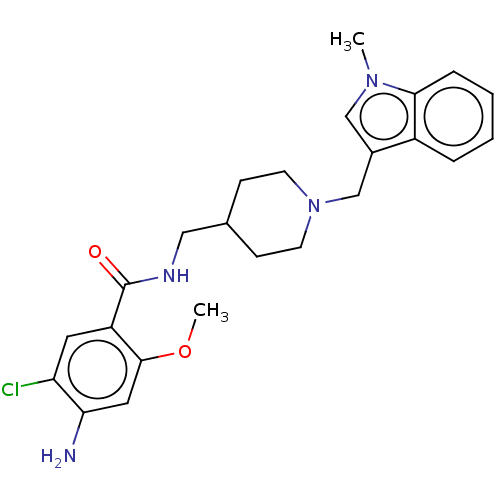
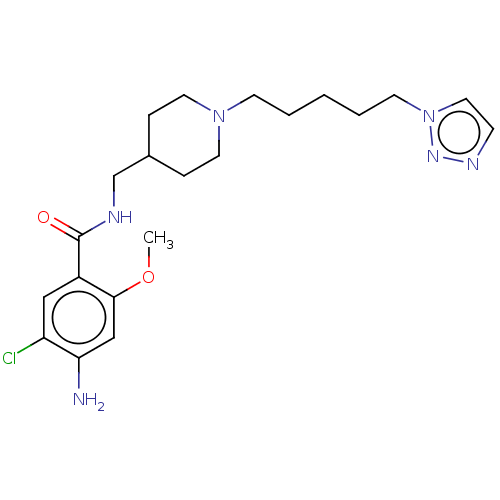
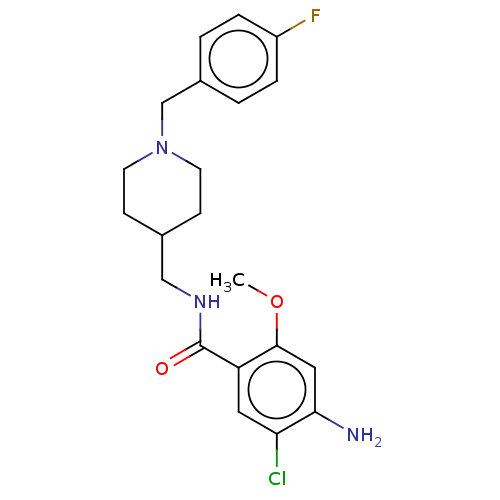
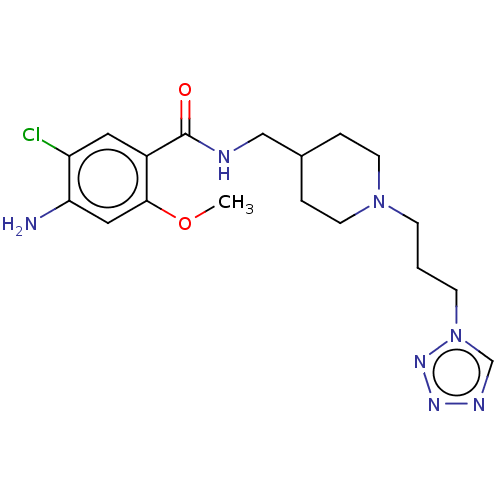
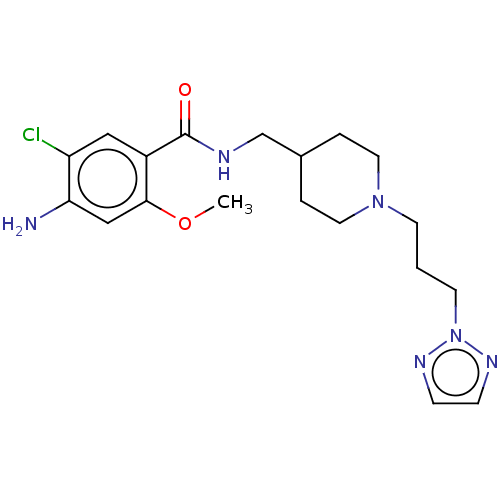
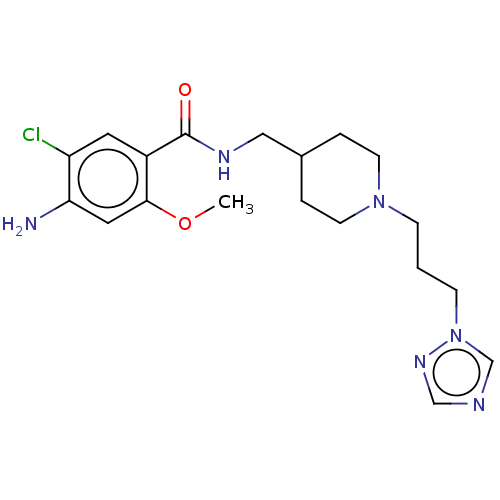
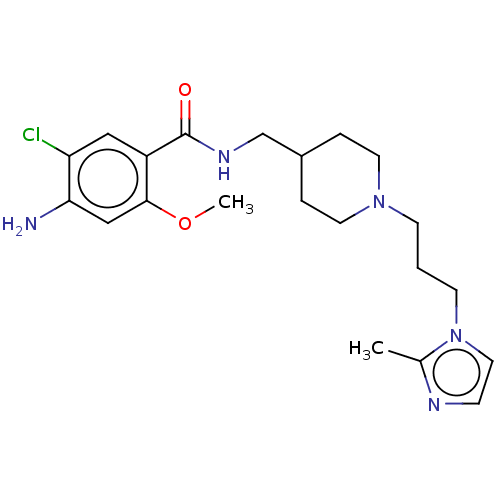
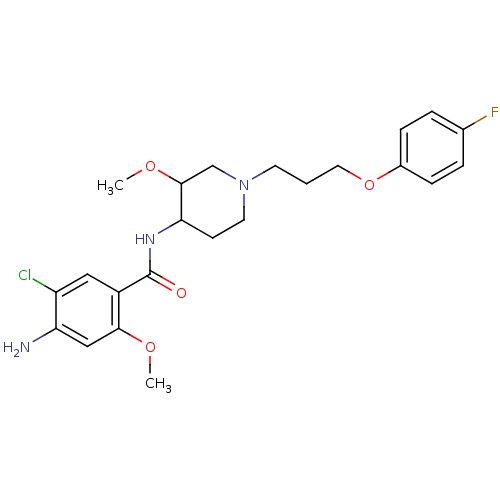
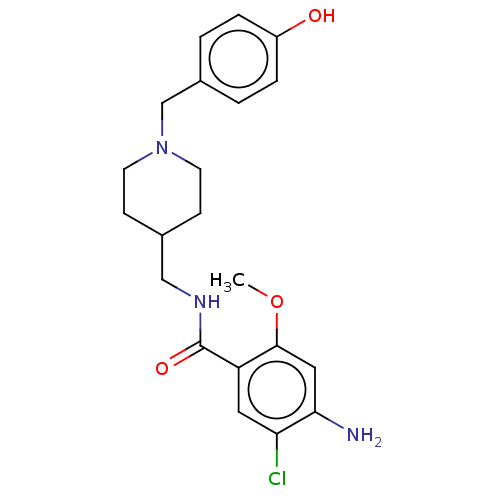
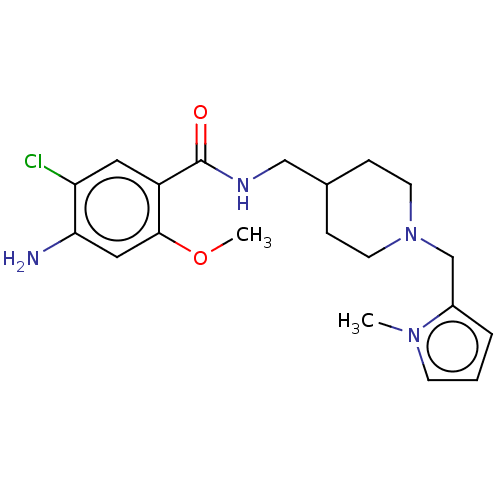
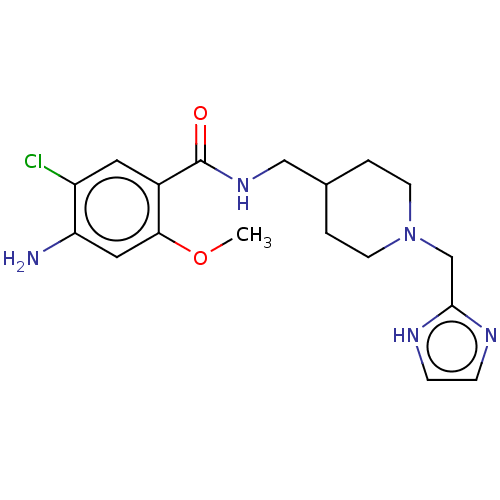
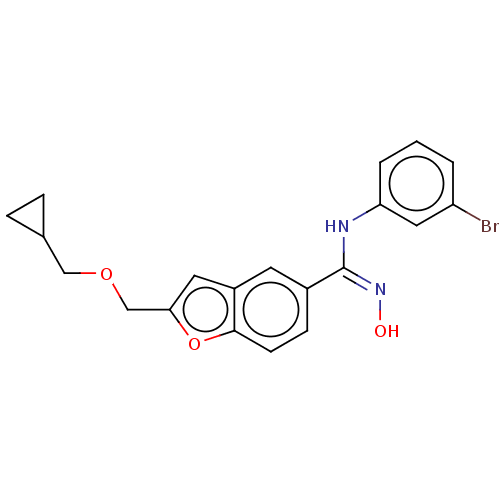
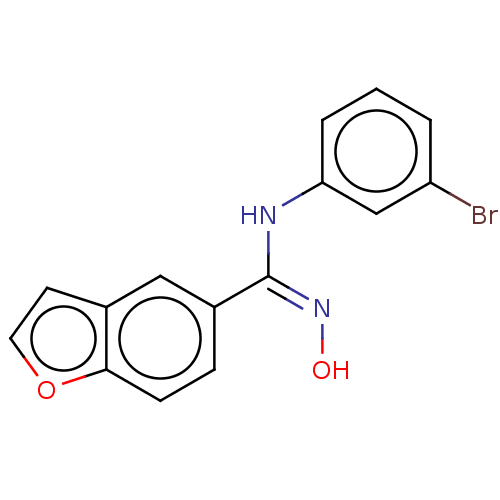
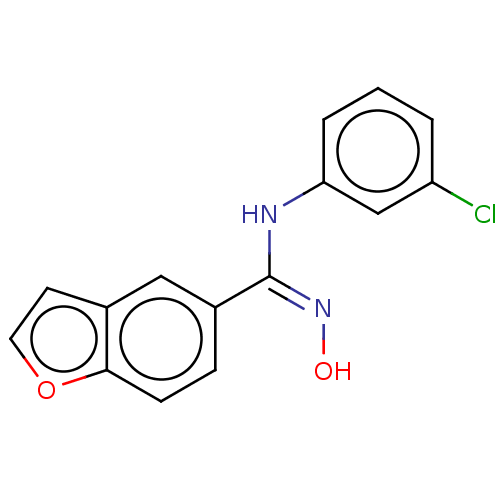
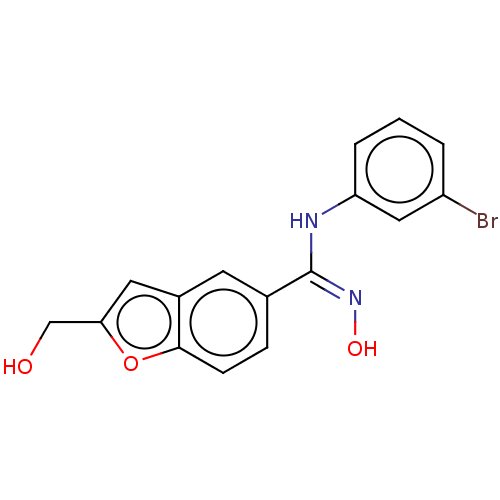
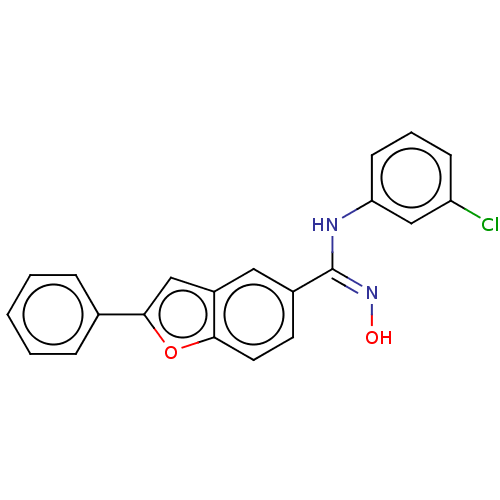
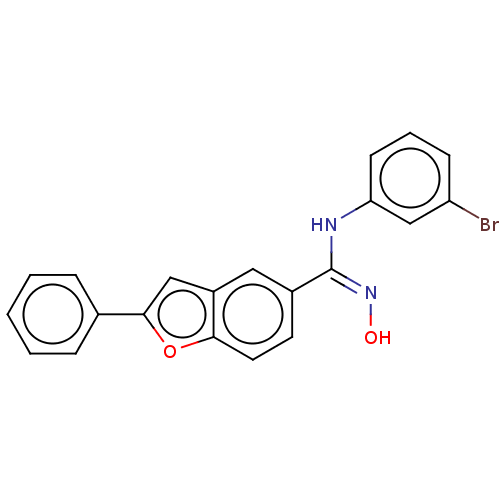
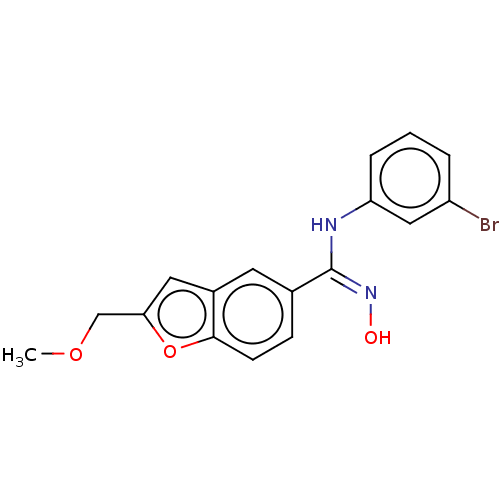
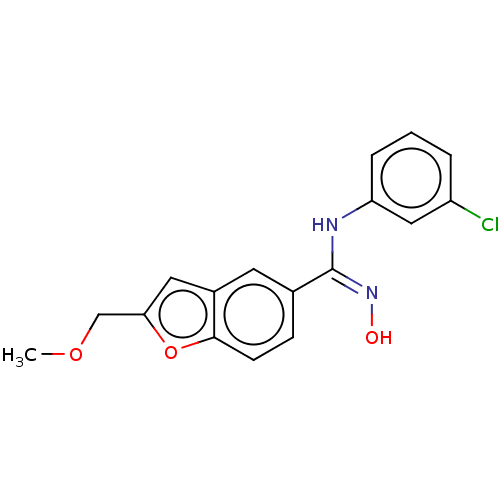
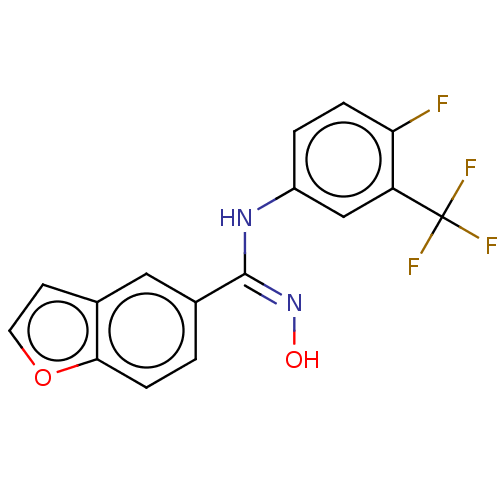
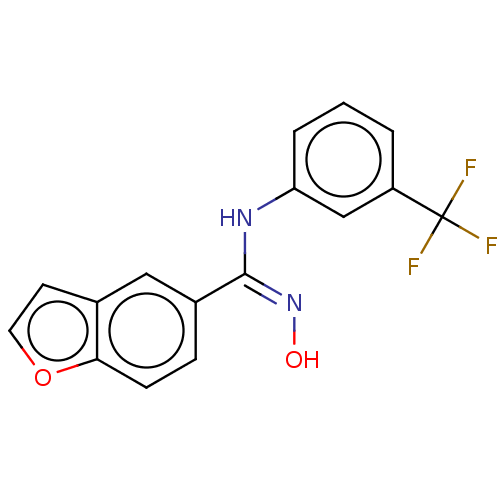
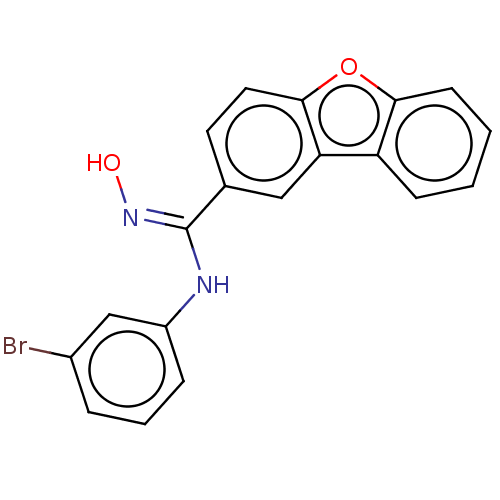
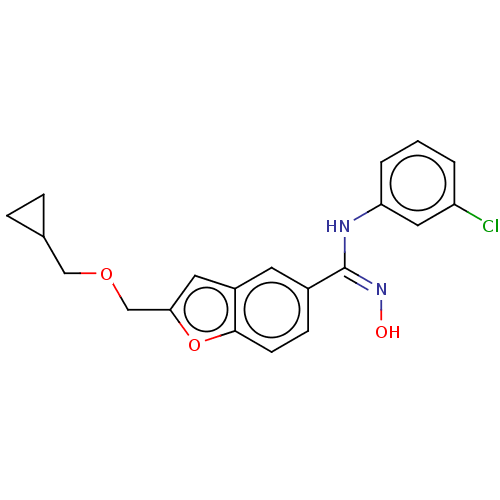
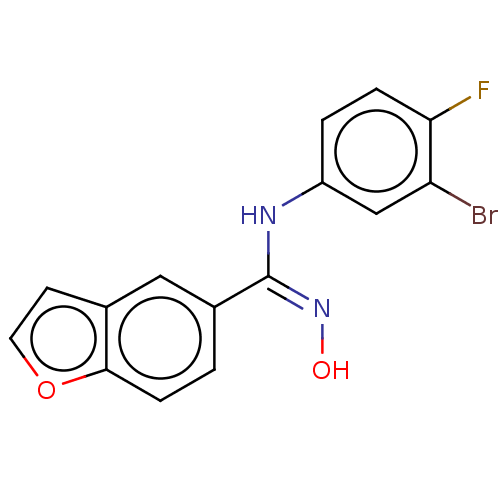
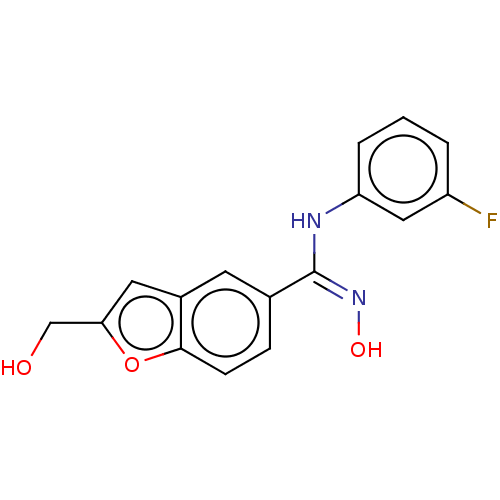
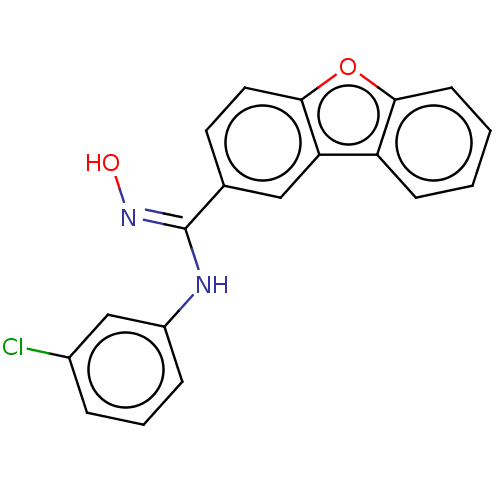
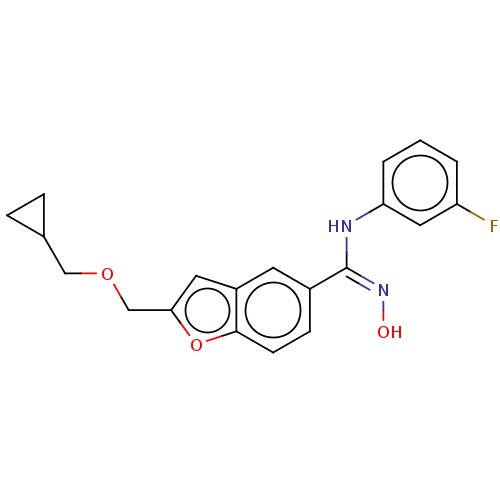
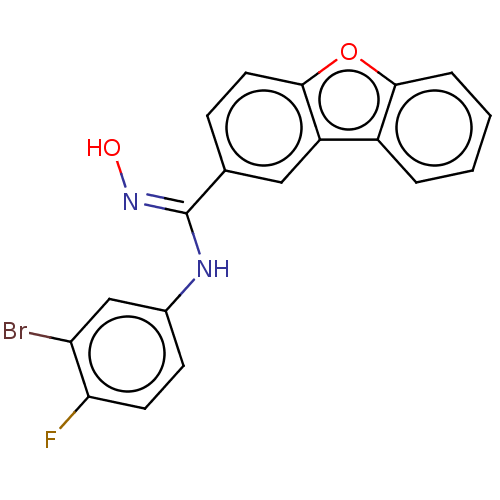
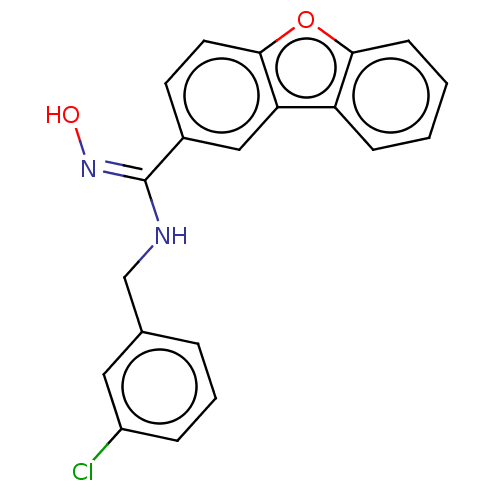
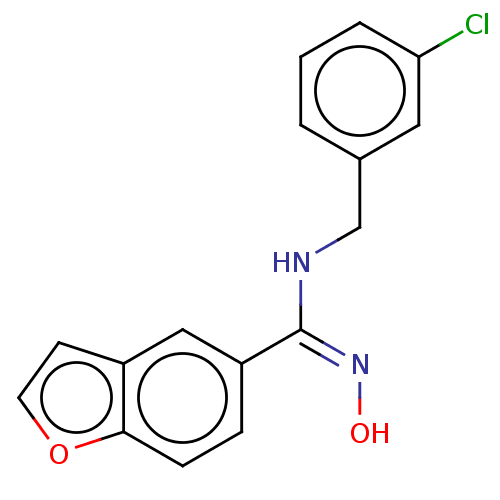
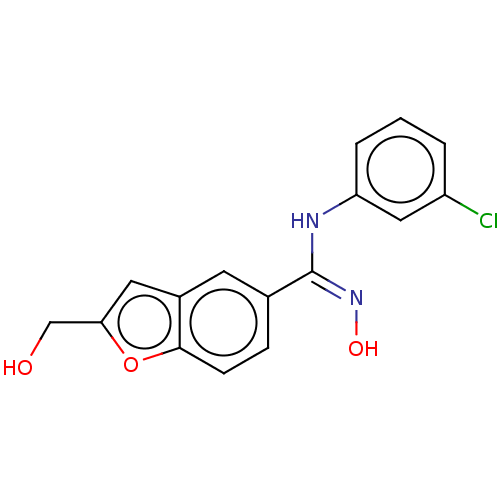
Affinity DataIC50: 0.00200nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0390nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0670nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0760nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.103nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.134nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.164nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.182nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.226nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.229nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.267nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.362nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.375nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.421nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 0.454nMAssay Description:The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Ildong Pharmaceutical
US Patent
Ildong Pharmaceutical
US Patent
Affinity DataIC50: 4.30E+3nMAssay Description:The inhibition of activity of hERG (human ether-a-go-go related gene) potassium channel by the compounds was evaluated by using an automatic whole-ce...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of IDO1 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair