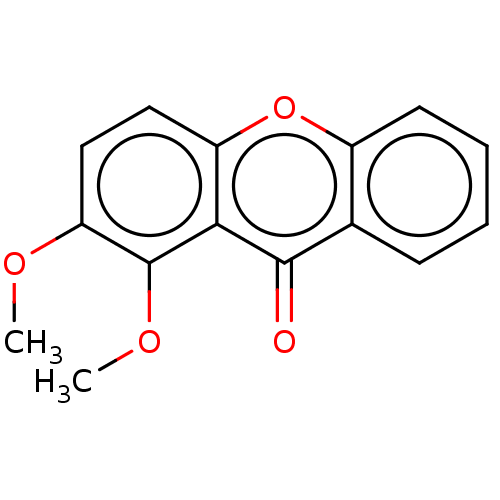
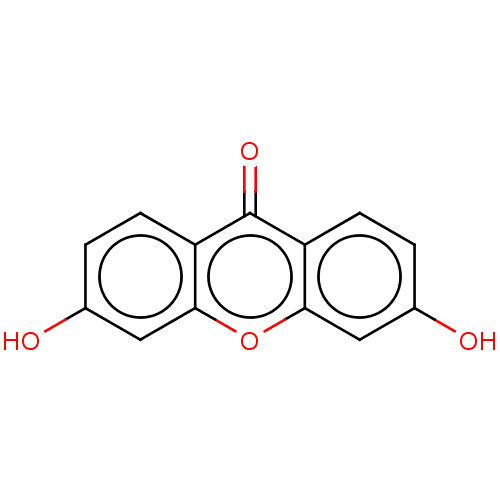
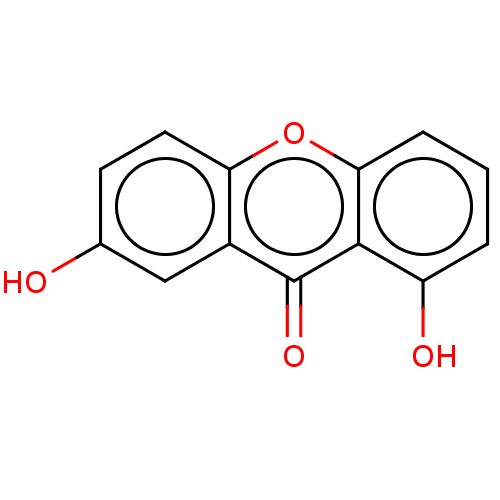
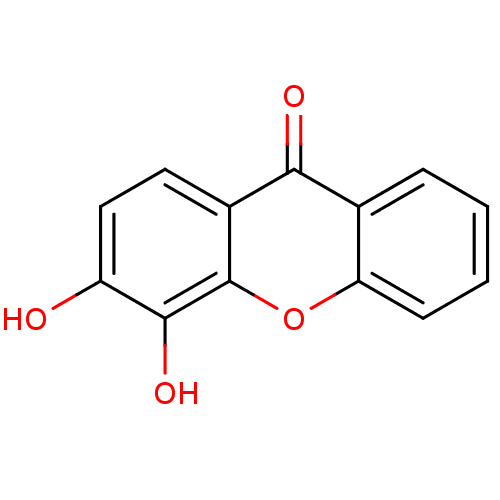
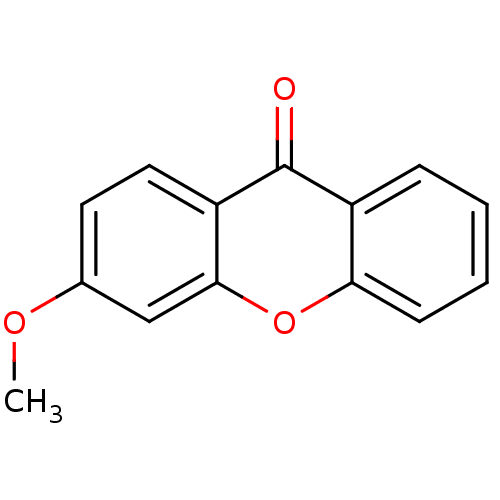
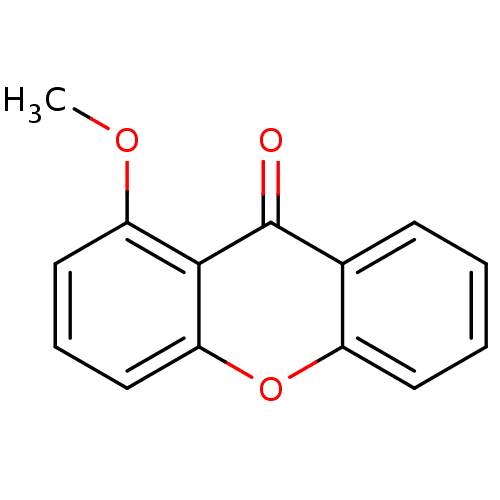
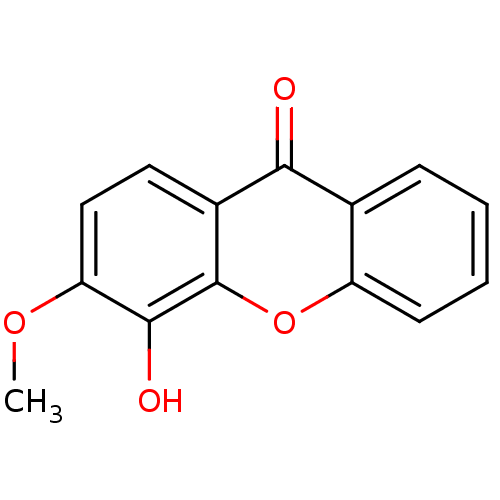
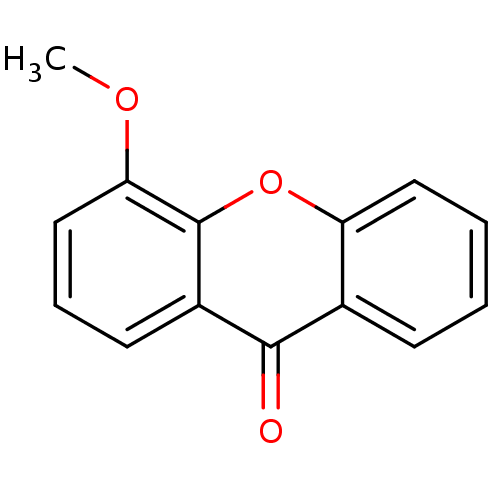
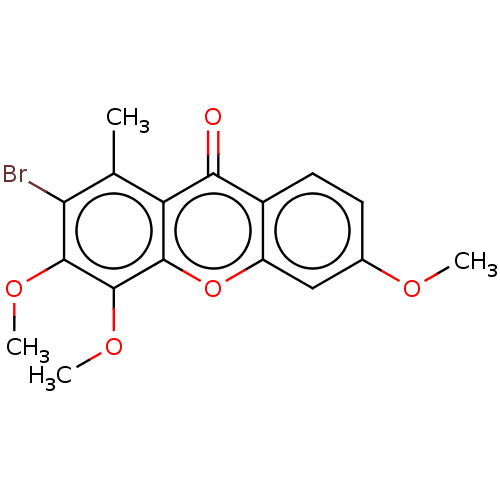
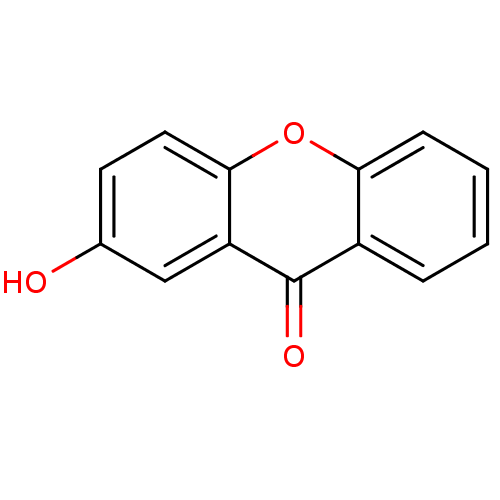
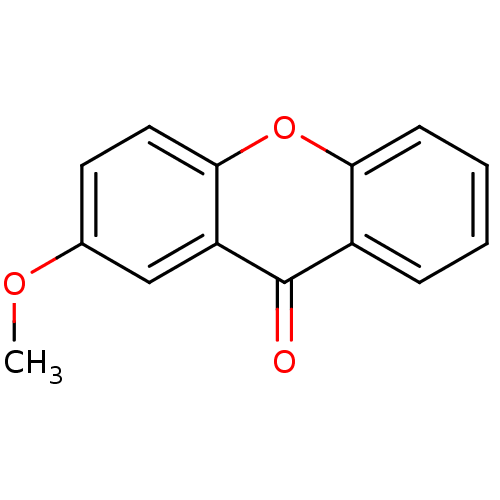
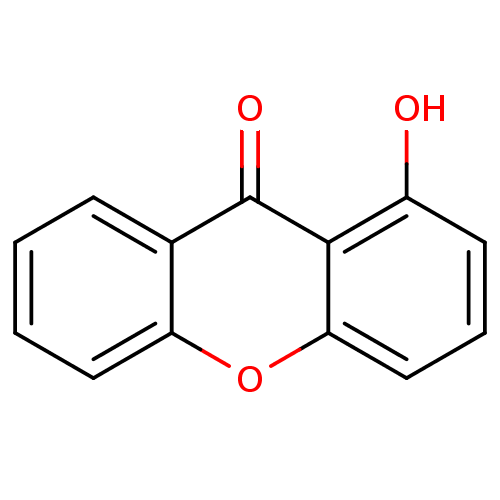
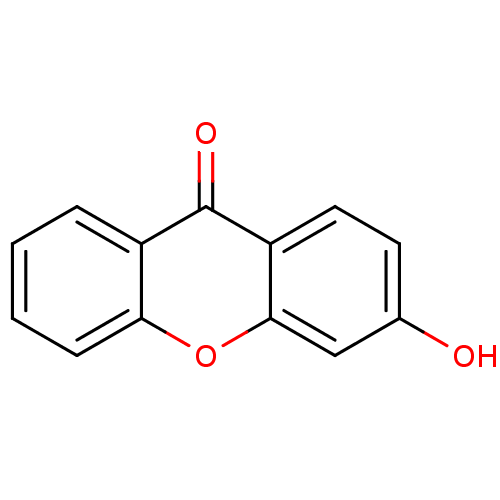
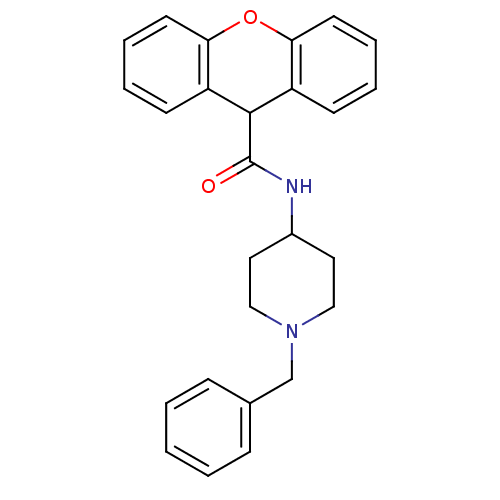
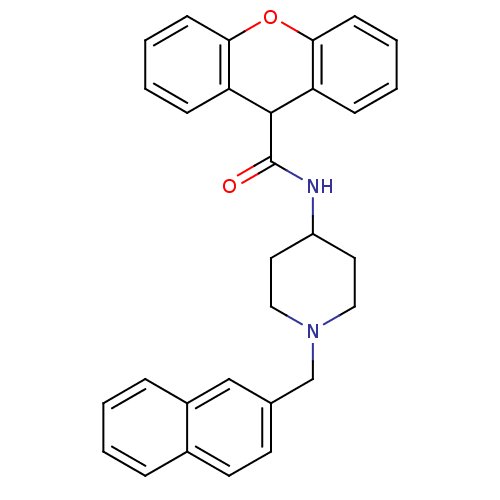
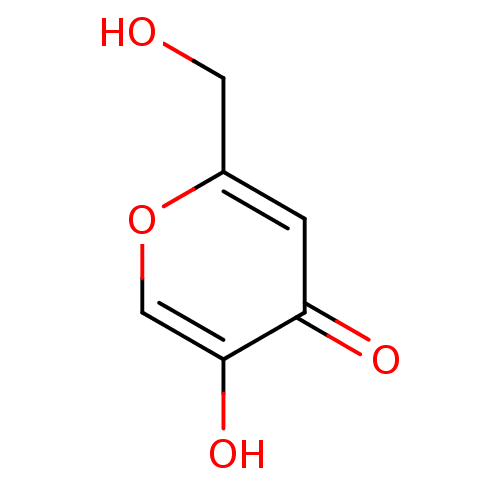
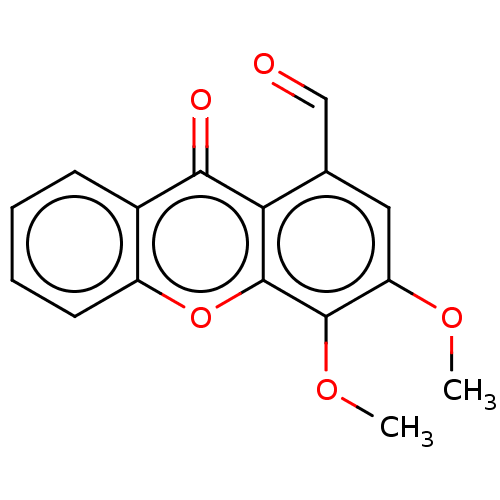
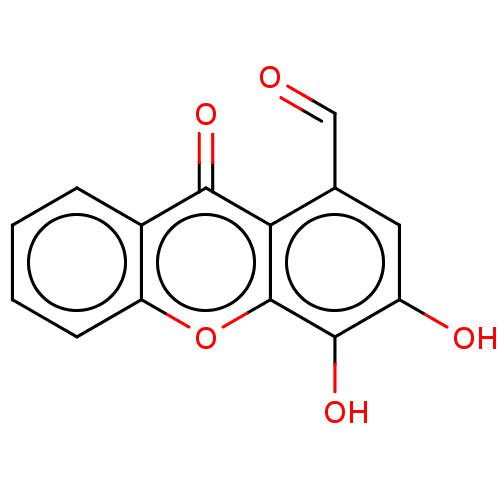
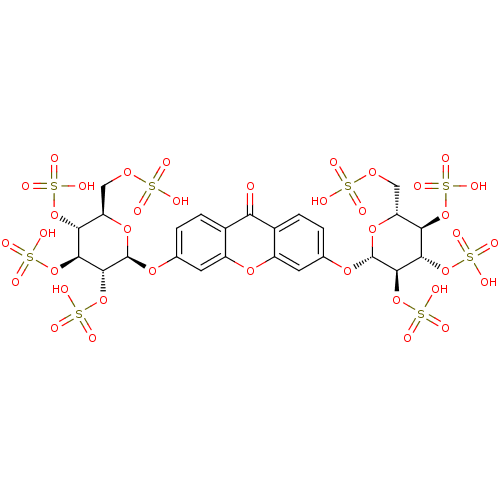
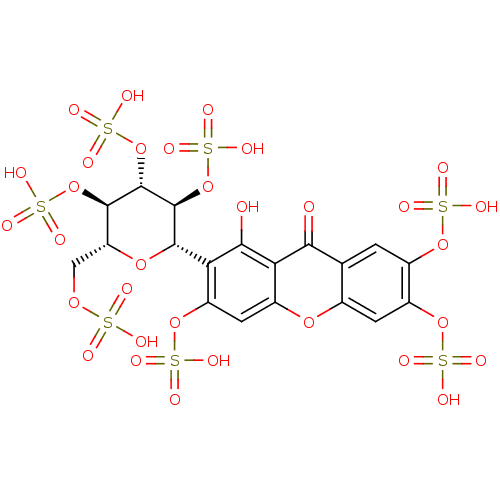
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Ciimar
Curated by ChEMBL
Ciimar
Curated by ChEMBL
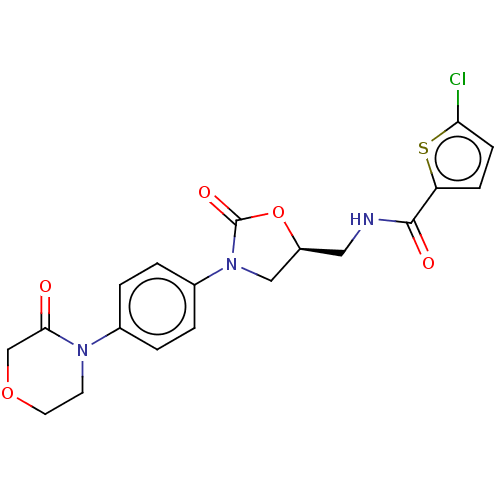
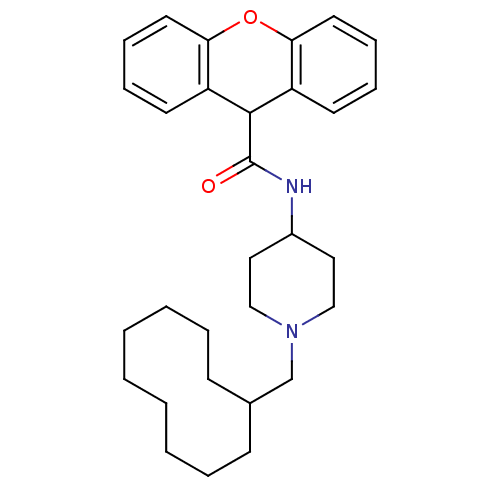
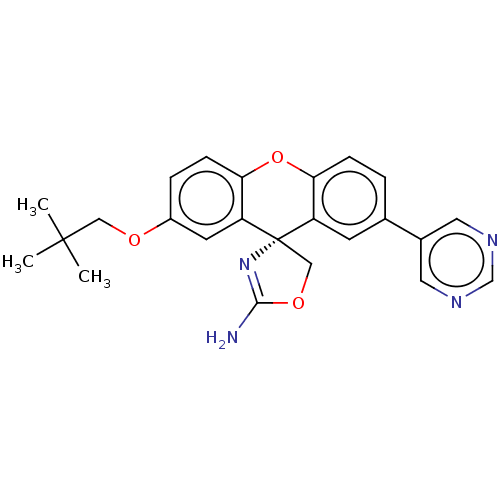
Affinity DataKi: 660nMAssay Description:Binding affinity to human ERG assessed as inhibition constantMore data for this Ligand-Target Pair
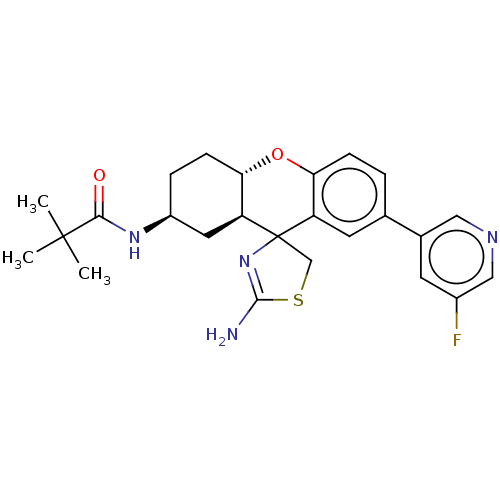
Affinity DataIC50: 1.10nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
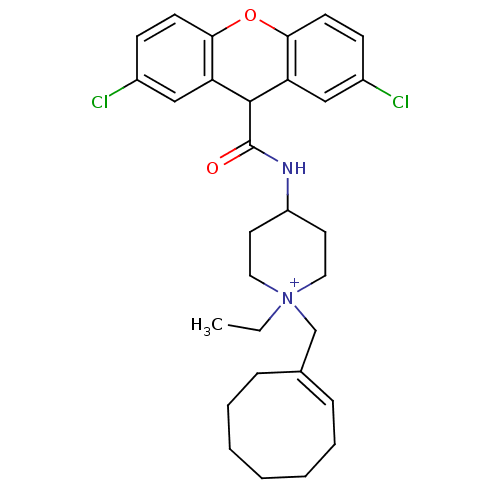
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
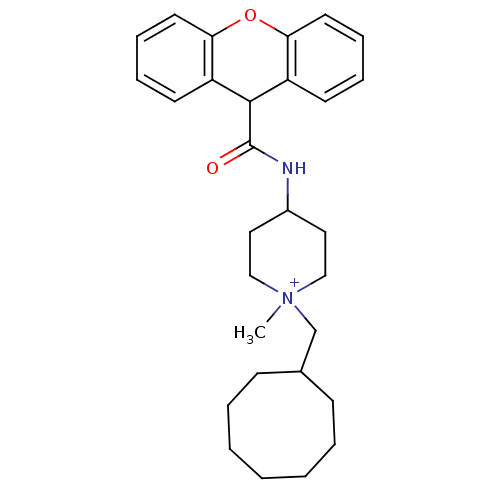
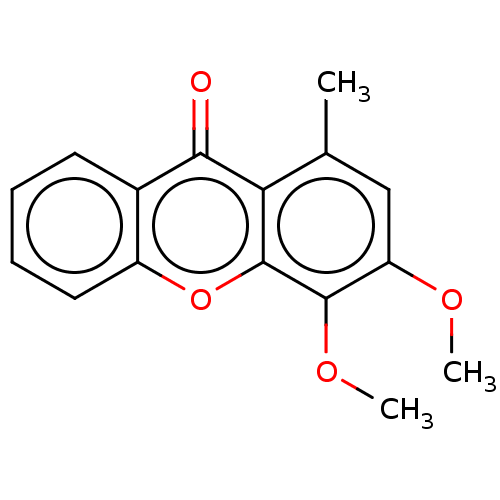
Affinity DataIC50: 4.59nMAssay Description:Inhibition of human factor 10a assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 2.24E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 3.01E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 3.28E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 3.37E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 3.81E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 4.02E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 4.42E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 4.52E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.12E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.12E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.14E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.14E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.33E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.57E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.29E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.46E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.47E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.83E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.84E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.93E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 9.26E+3nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.28E+4nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 2.91E+4nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8.94E+4nMAssay Description:Inhibition of Tyrosinase (unknown origin) using tyrosine as substrate incubated for 20 mins followed by substrate addition and measured every 10 mins...More data for this Ligand-Target Pair
TargetAntithrombin-III/Coagulation factor X(Homo sapiens (Human))
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
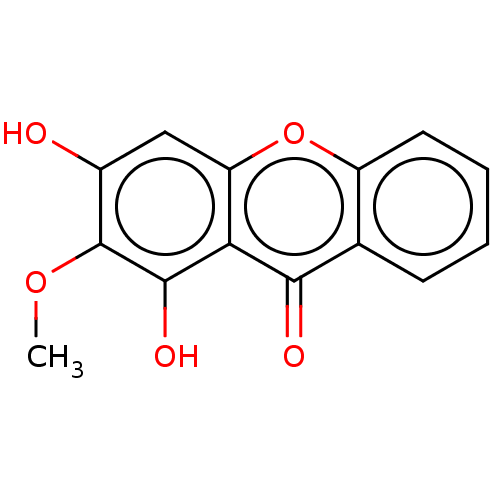
Affinity DataIC50: 1.90E+5nMAssay Description:Inhibition of human factor 10a/antithrombin 3 assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
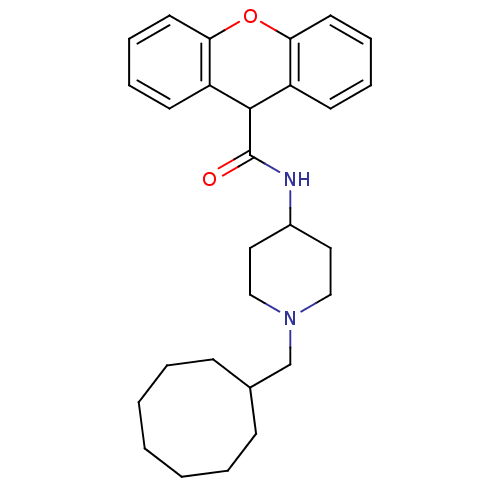
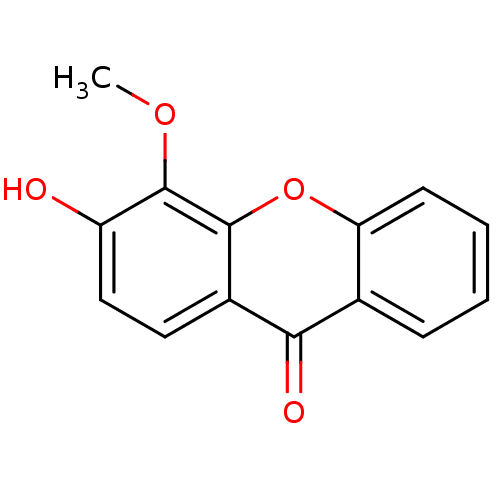
Affinity DataIC50: 7.40E+5nMAssay Description:Inhibition of human factor 10a assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
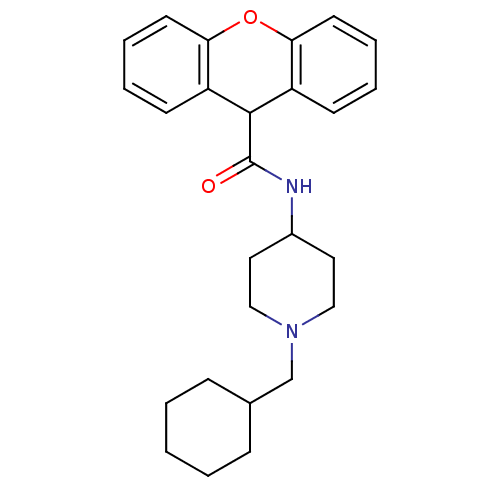
Affinity DataIC50: 9.70E+5nMAssay Description:Inhibition of human factor 10a assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysisMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)