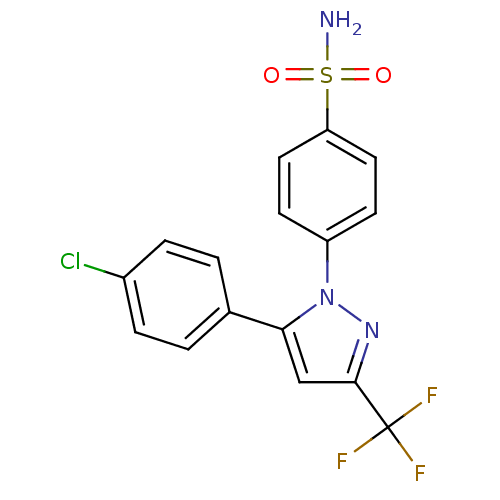
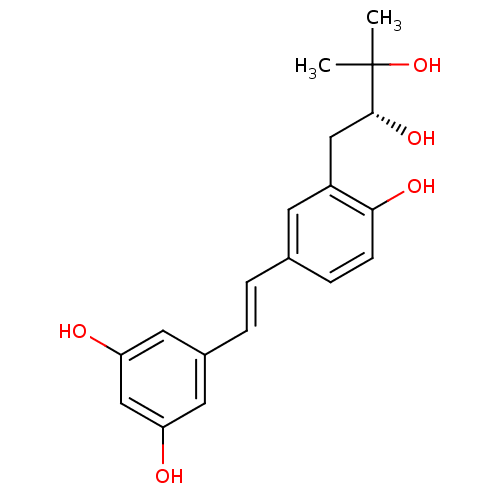
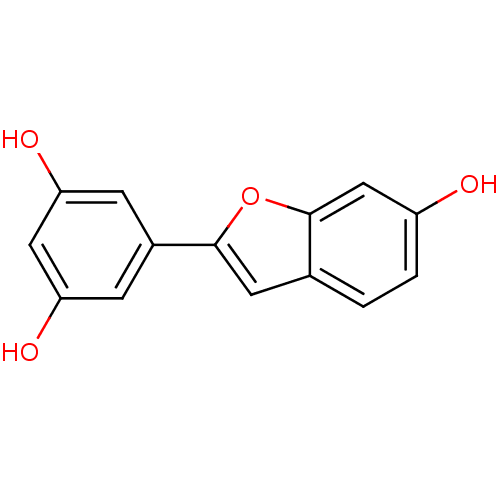
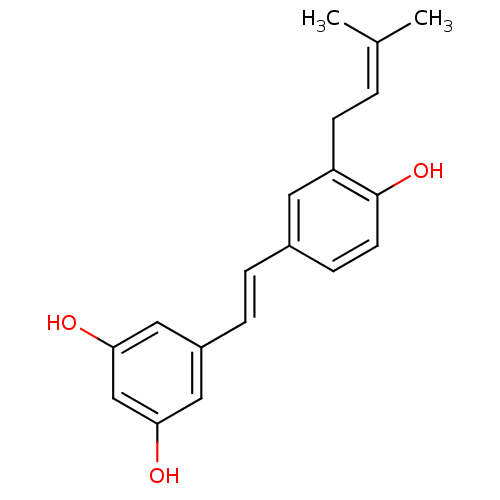
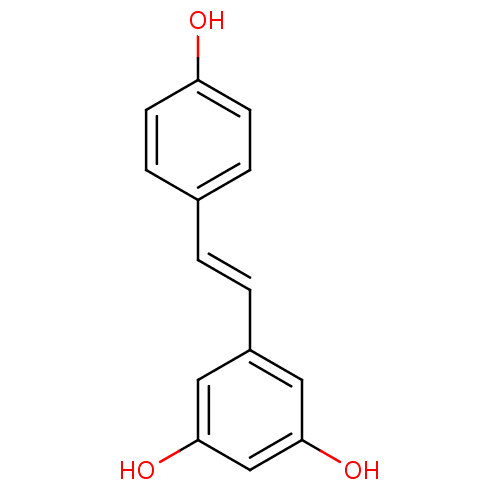
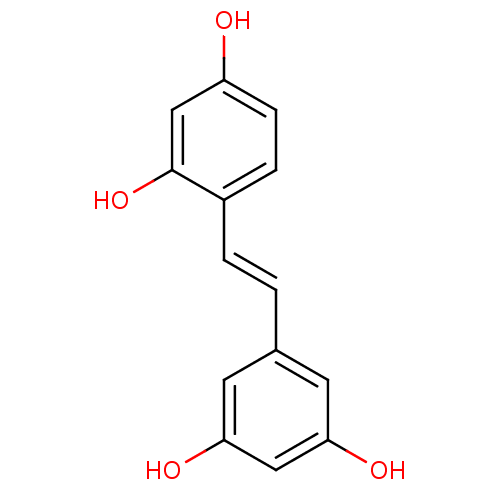
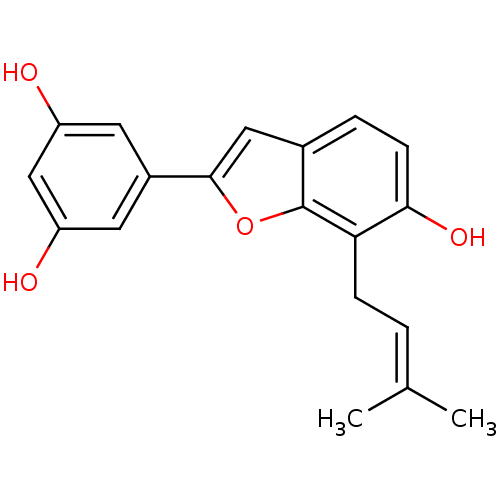
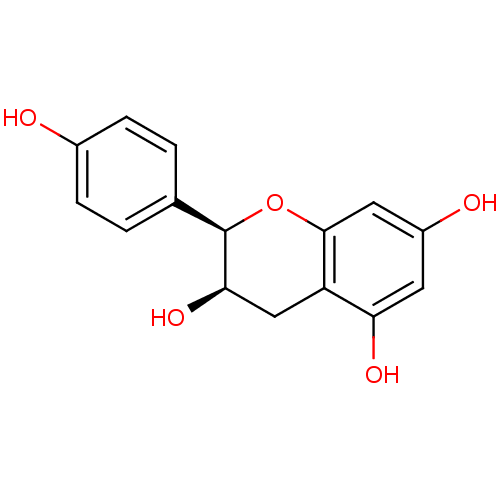
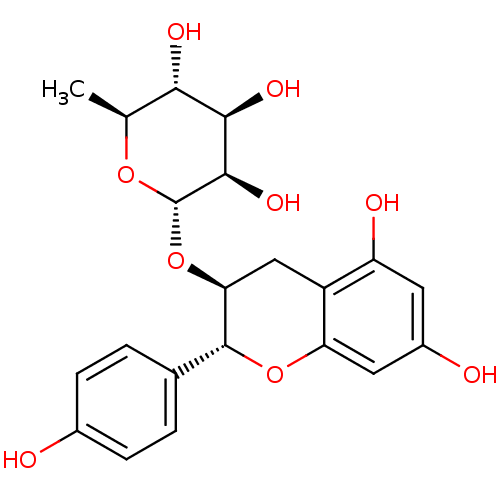
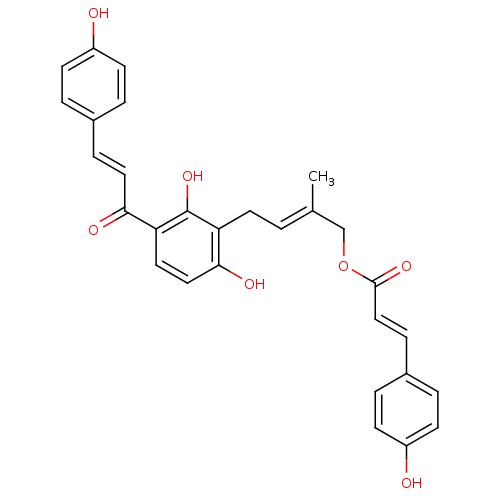
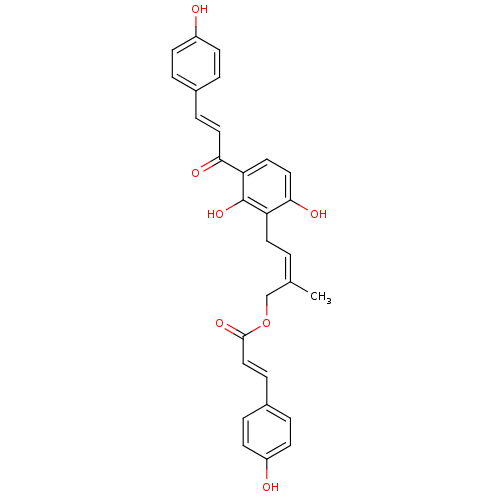
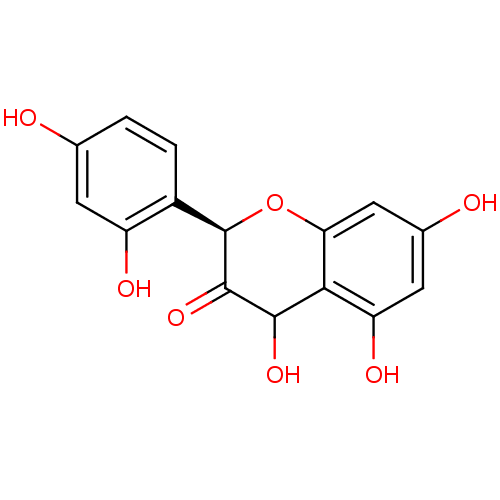
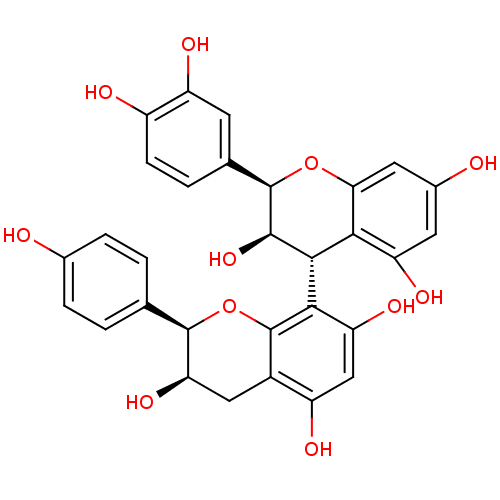
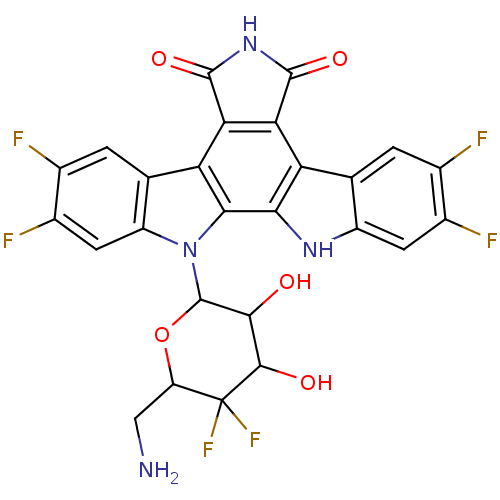
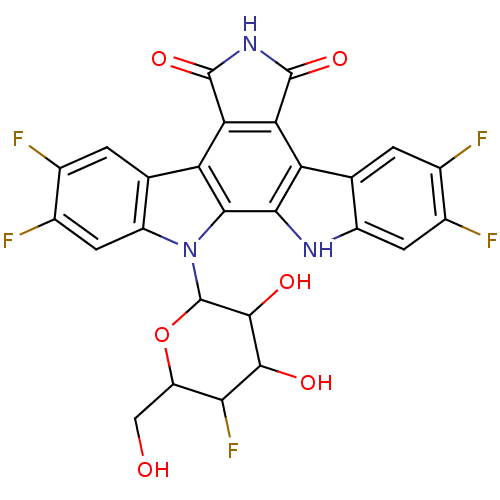
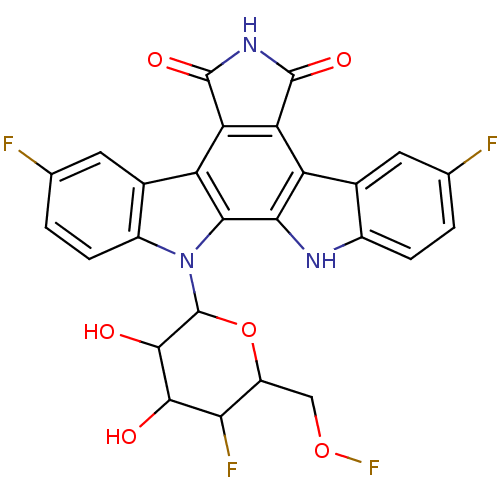
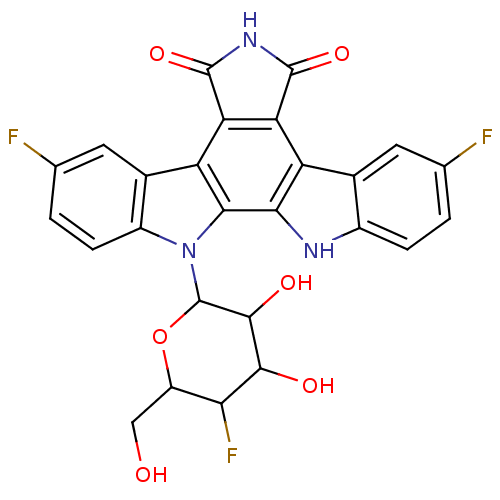
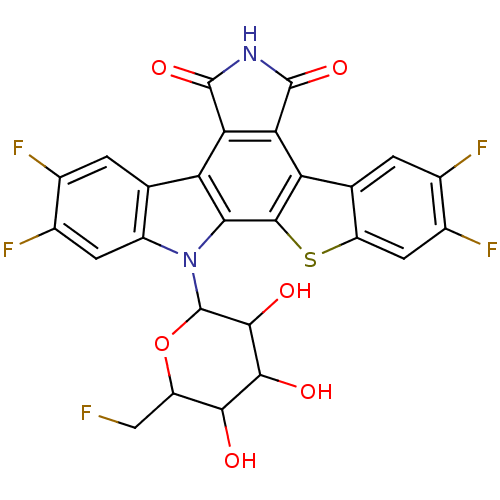
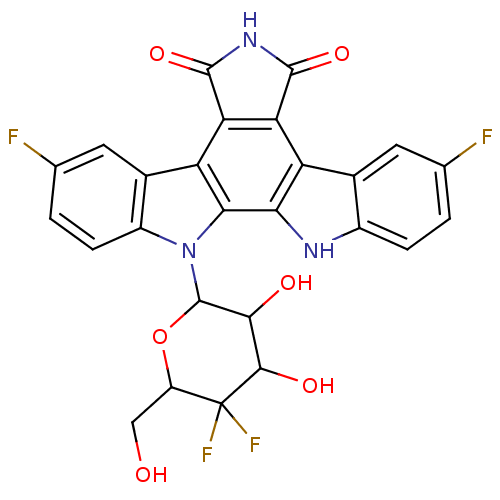
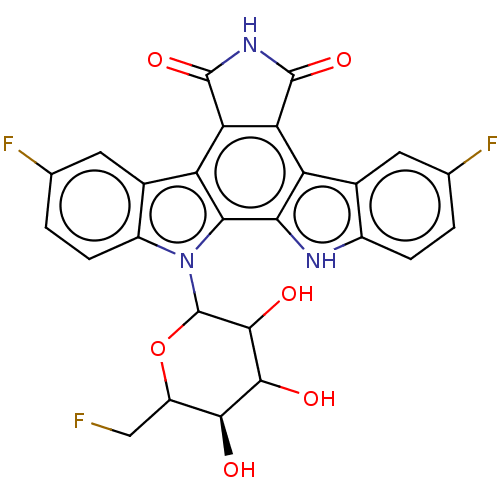
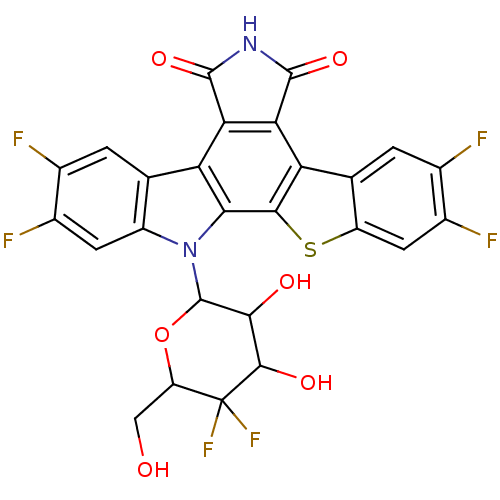
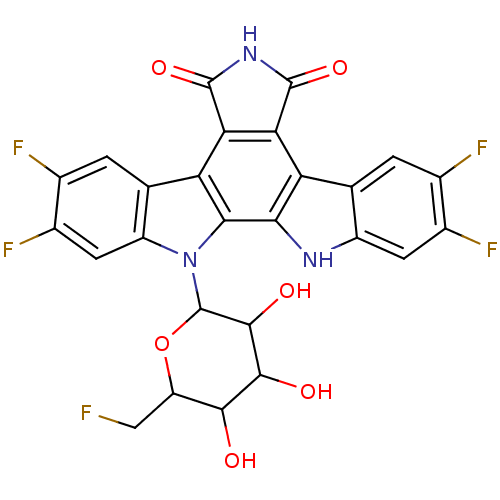
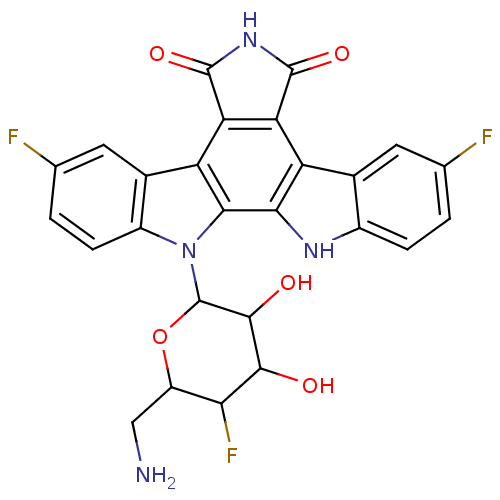
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 8.30E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.17E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.39E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 2.23E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.14E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.18E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.67E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.84E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 4.64E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 6.71E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 6.97E+4nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.09E+5nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 6.40nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 32nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 120nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 65.6nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 17.6nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 704nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 240nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 12.8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 76.8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 80nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 480nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 11.2nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 38.4nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 67.2nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair