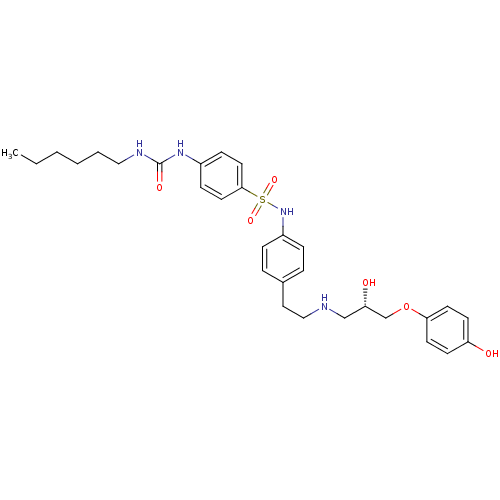
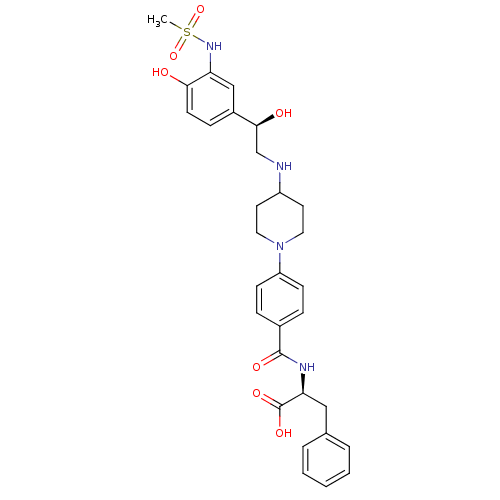
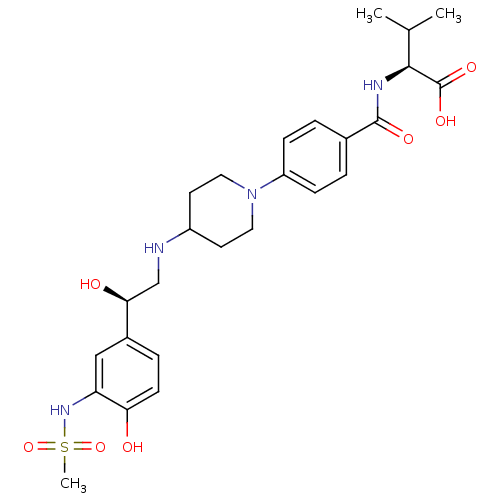
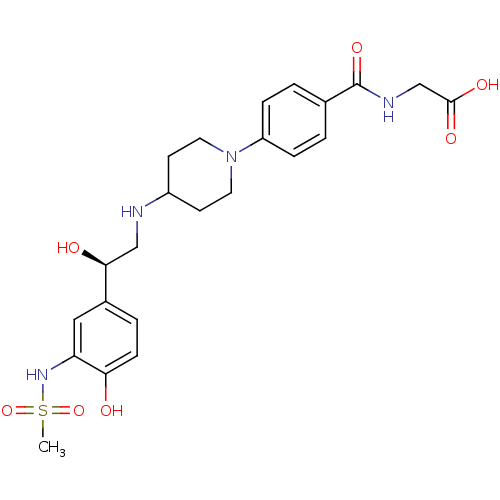
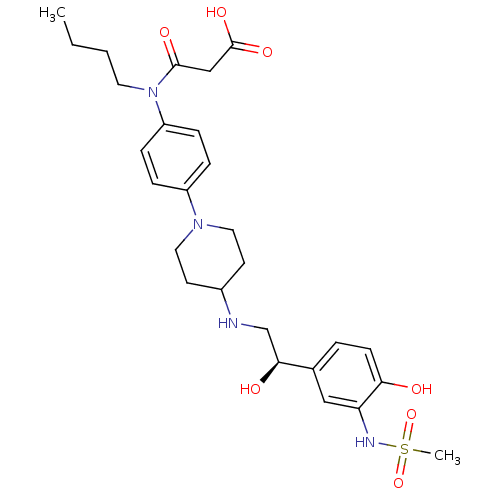
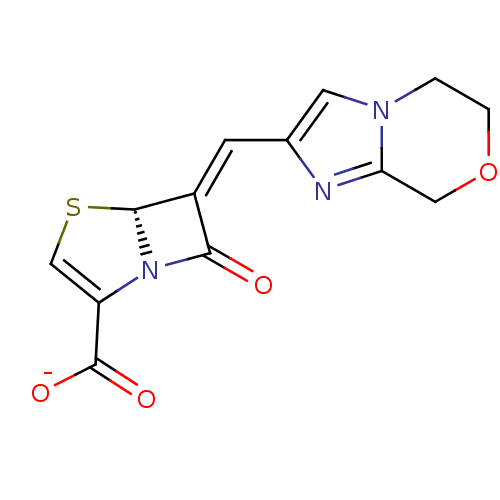
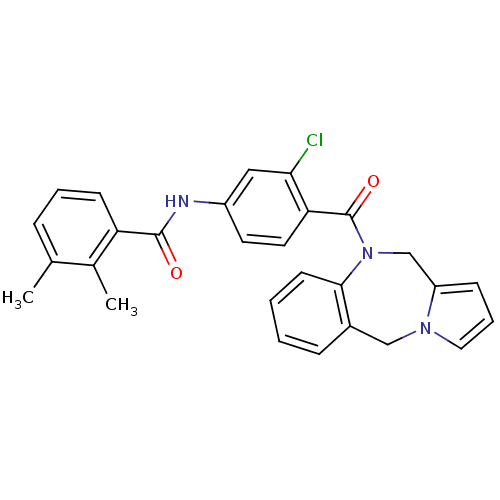
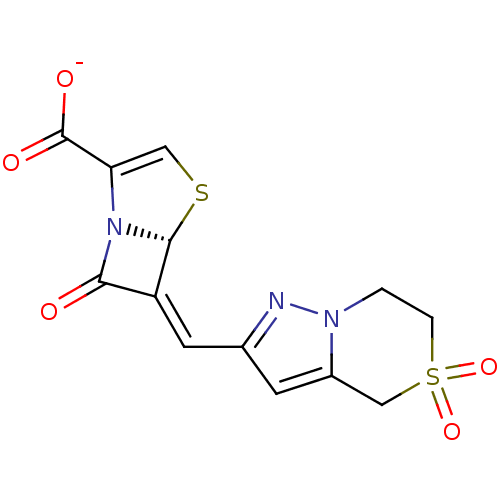
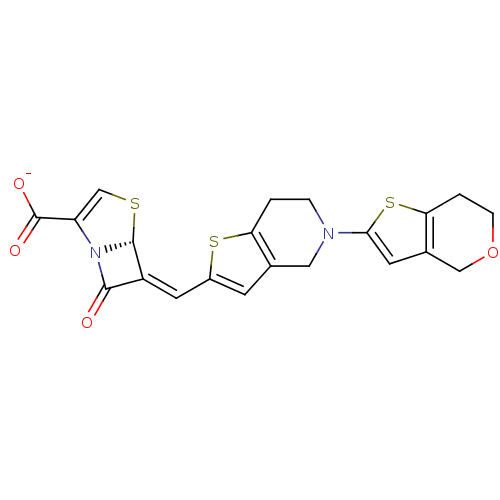
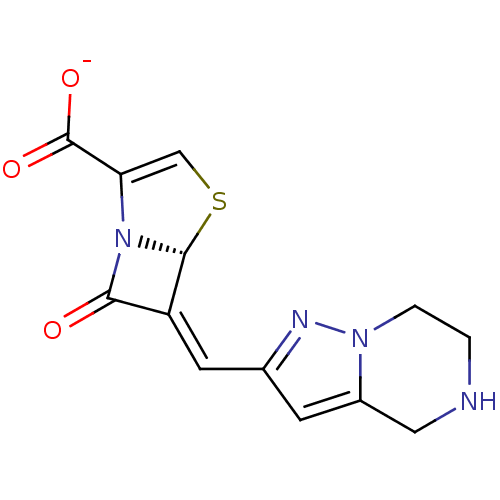
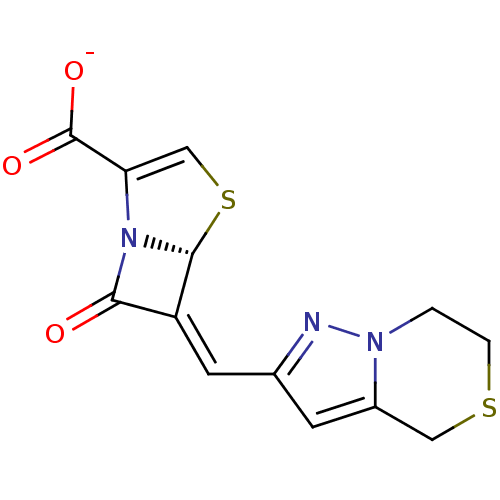
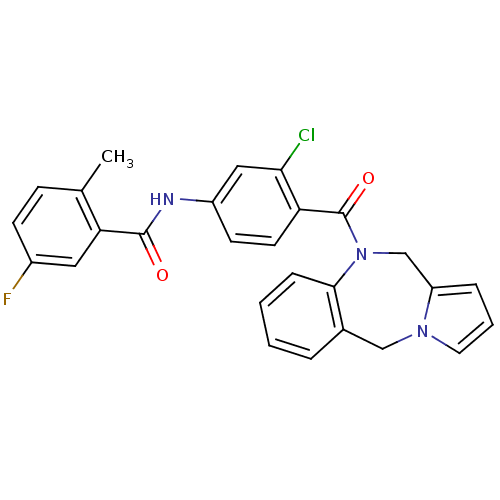
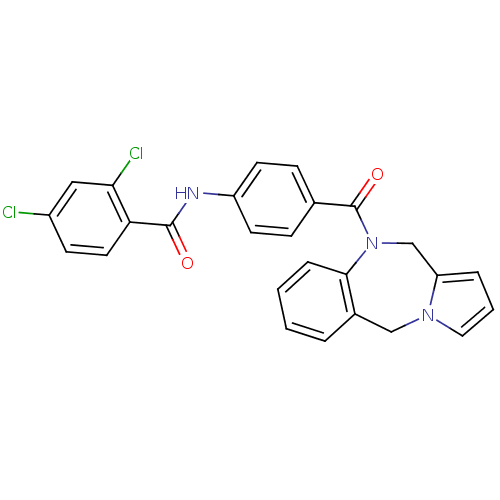
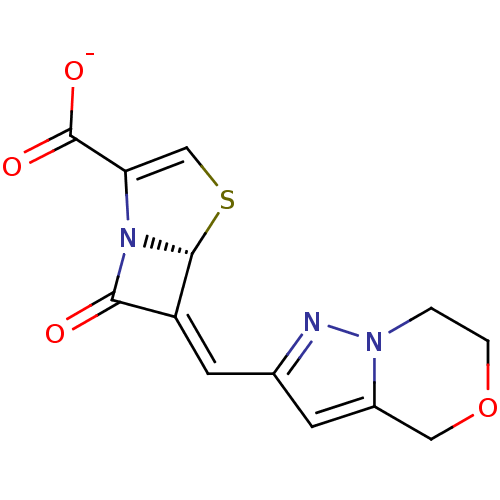
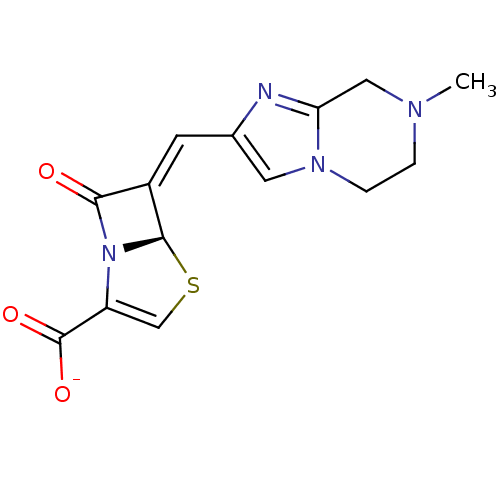
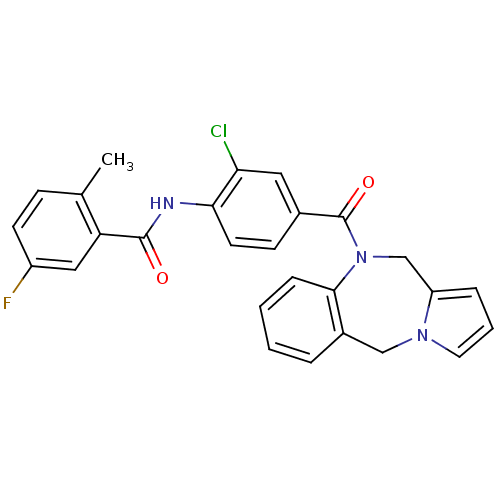
Affinity DataKi: 0.100nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 1.16E+4nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 7.92E+4nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla.More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranesMore data for this Ligand-Target Pair