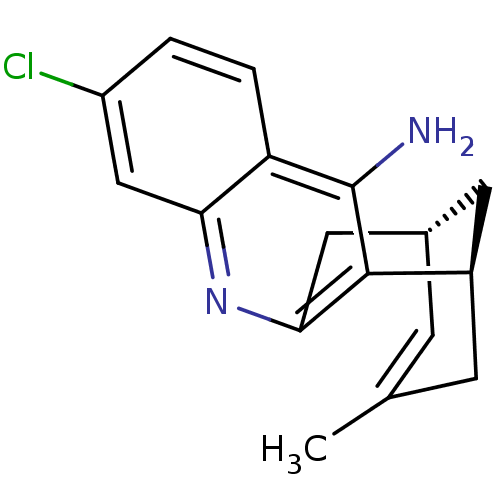
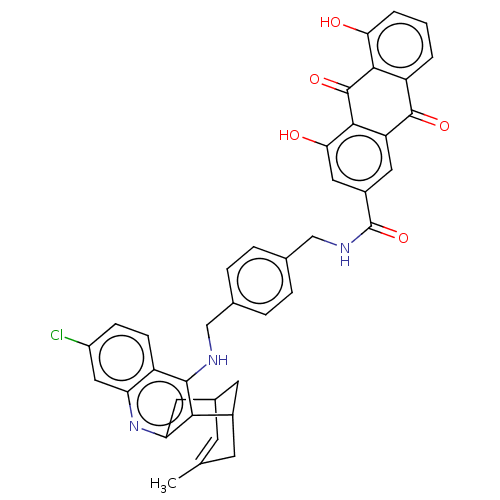
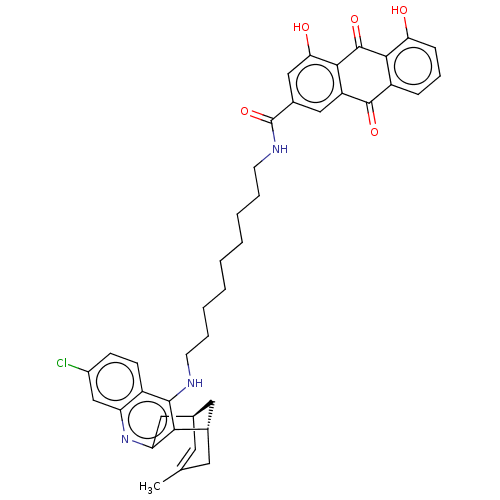
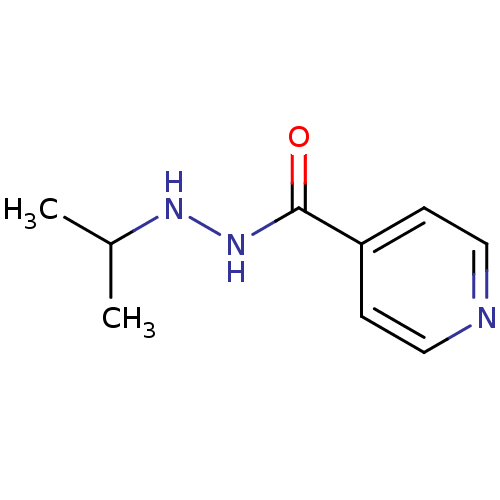
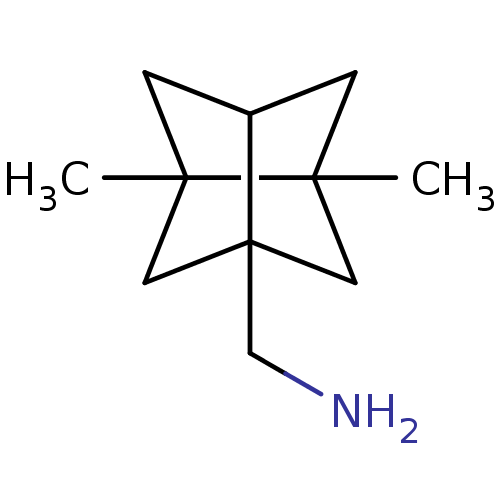
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
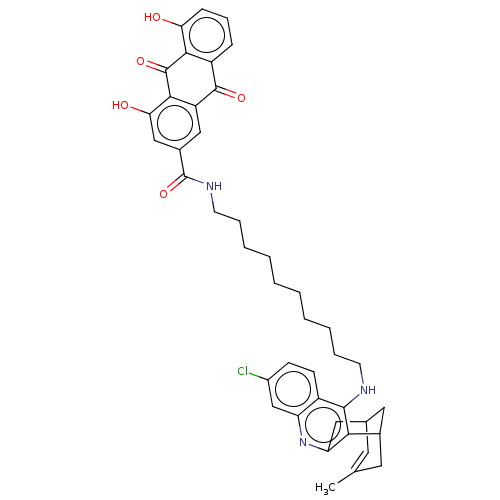
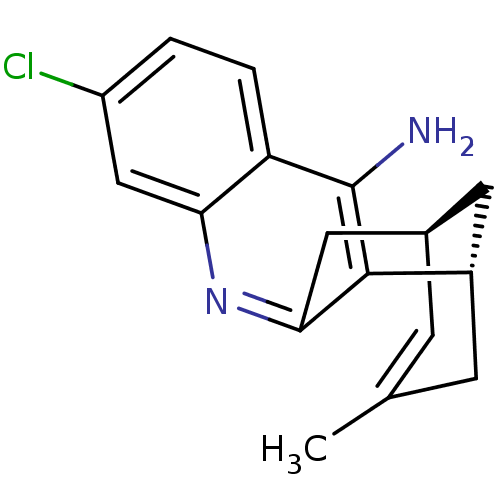
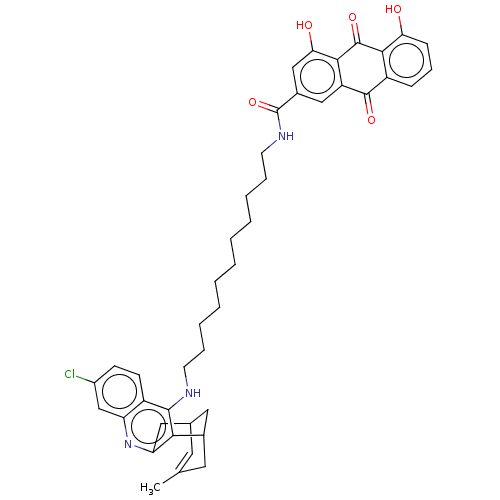
Affinity DataIC50: 0.0600nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 0.0800nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 9.40nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 13.6nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 16.3nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu...More data for this Ligand-Target Pair
Affinity DataIC50: 17.4nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 18.2nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 32.6nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 46.7nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 60nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 88.6nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 98.9nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 181nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 222nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 265nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 373nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 513nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 620nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universitat De Barcelona
US Patent
Universitat De Barcelona
US Patent
Affinity DataIC50: 637nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 645nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 980nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+3nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+3nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa...More data for this Ligand-Target Pair
Affinity DataIC50: 2.79E+3nMAssay Description:Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.93E+3nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMT: 2°CAssay Description:β-Secretase (BACE-1, Sigma) inhibition studies were performed
by employing a peptide mimicking APP sequence as substrate
(methoxycoumarin-Ser-...More data for this Ligand-Target Pair
Affinity DataIC50: 6.56E+3nMAssay Description:Inhibition of human recombinant MAOA expressed in baculovirus-infected BTI insect cells assessed as conversion of p-tyramine into p-hydroxyphenyl-ace...More data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa...More data for this Ligand-Target Pair
Affinity DataIC50: 7.54E+3nMAssay Description:Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu...More data for this Ligand-Target Pair
Affinity DataIC50: 9.26E+3nMAssay Description:Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Universidad Nacional AutóNoma De MéXico
Curated by ChEMBL
Universidad Nacional AutóNoma De MéXico
Curated by ChEMBL
Affinity DataIC50: 9.94E+3nMAssay Description:Inhibition of human DNA topoisomerase 2-mediated Crithidia fasciculata kDNA decatenation using ethidium bromide staining by agarose gel electrophores...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and
(+)-(Ib) was evaluated spectrophotometrically at 25° C. by the
method of Ellma...More data for this Ligand-Target Pair