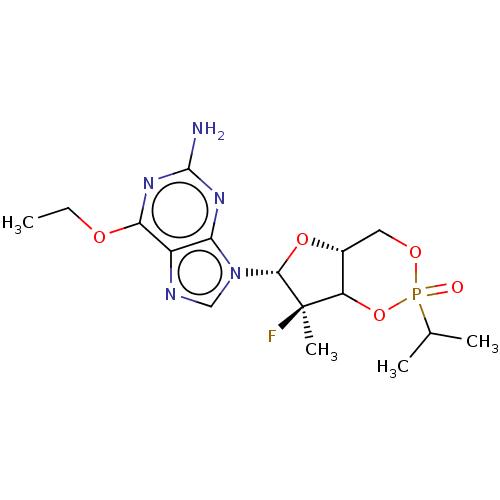
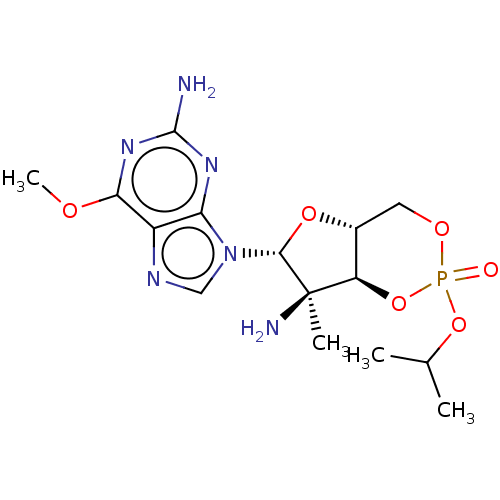
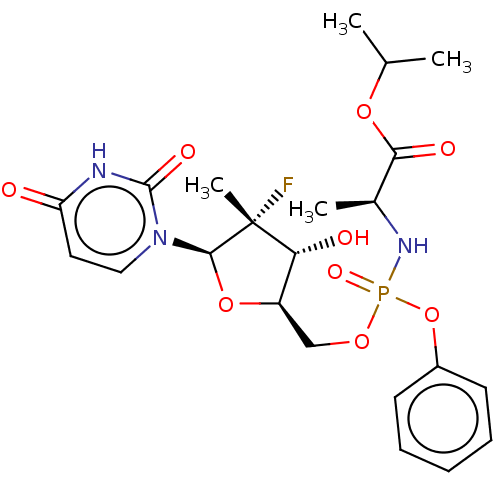
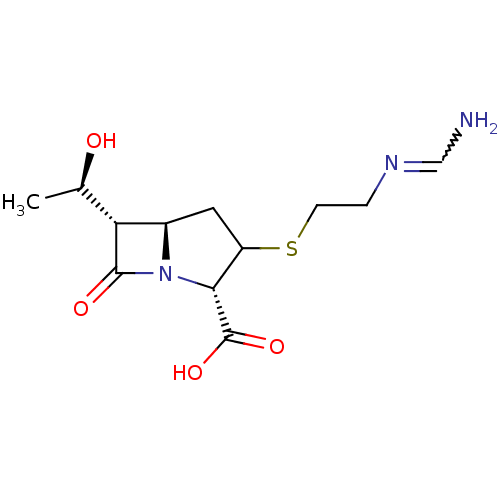
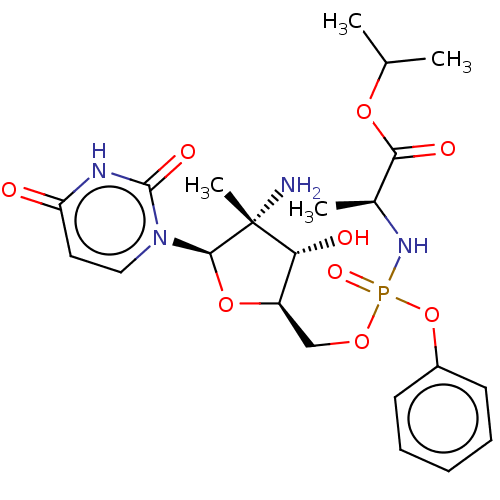
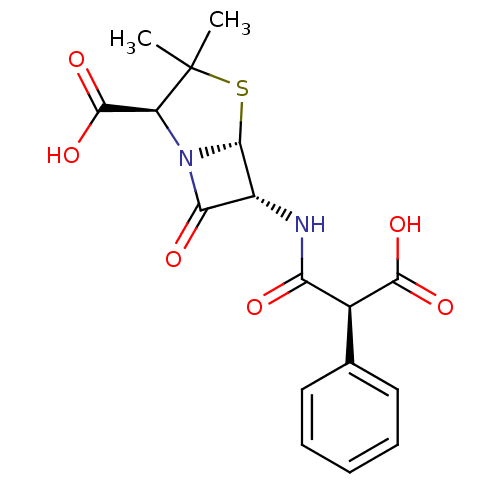
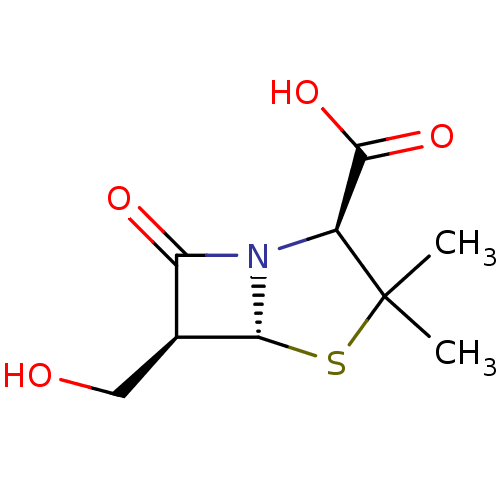
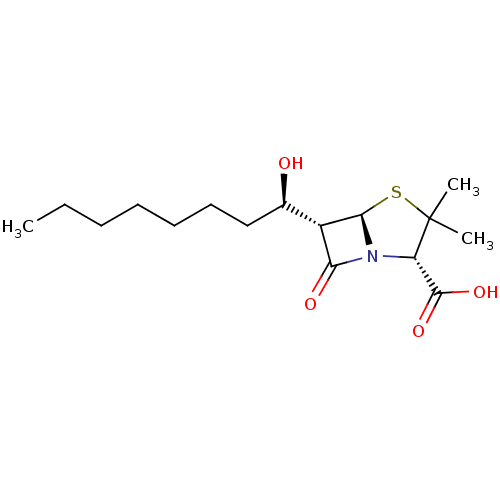
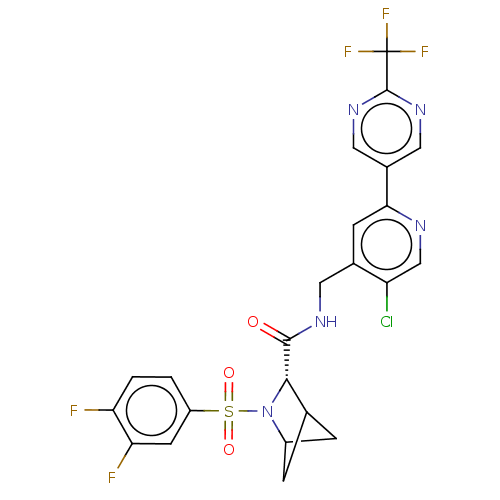
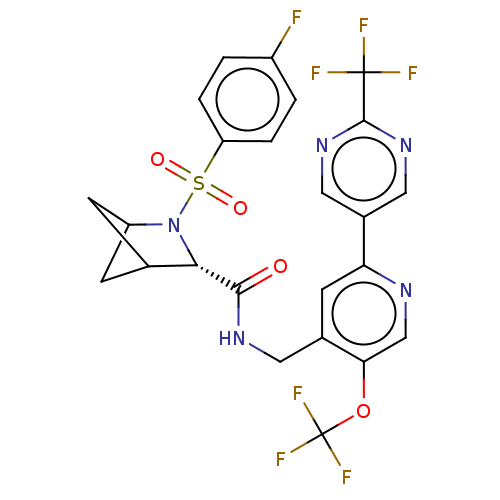
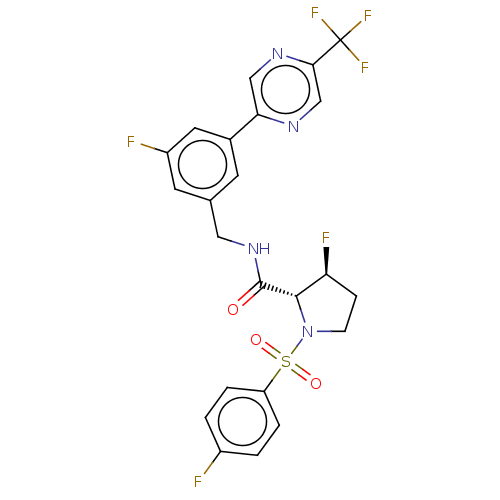
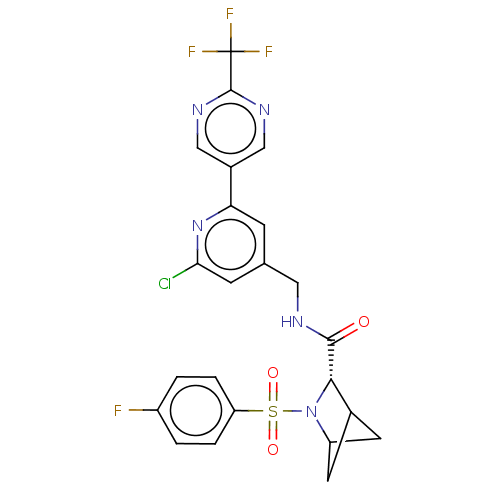
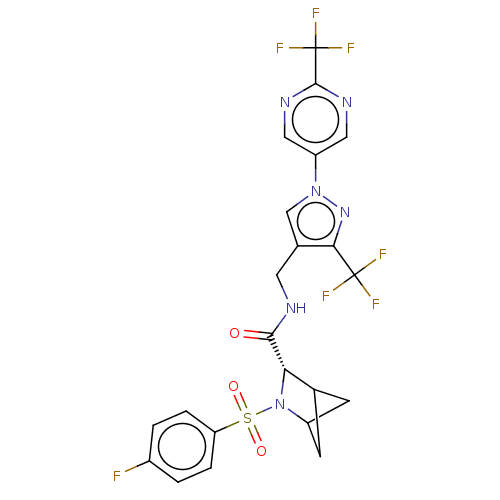
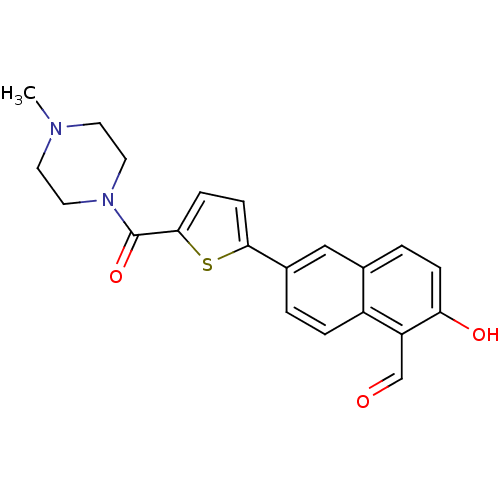
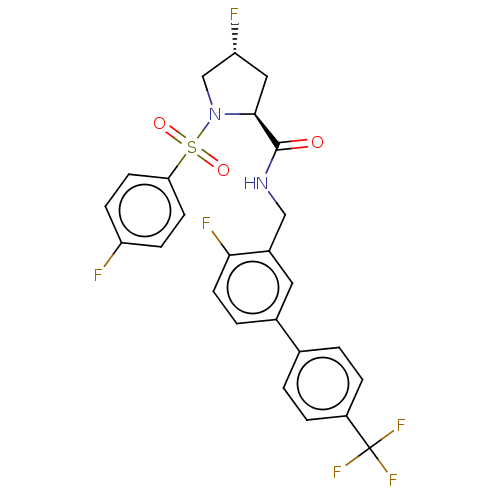
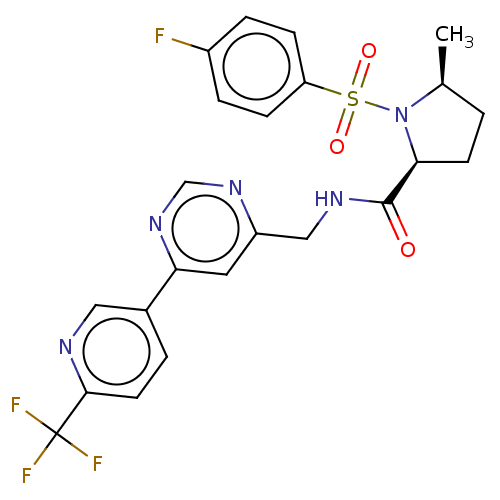
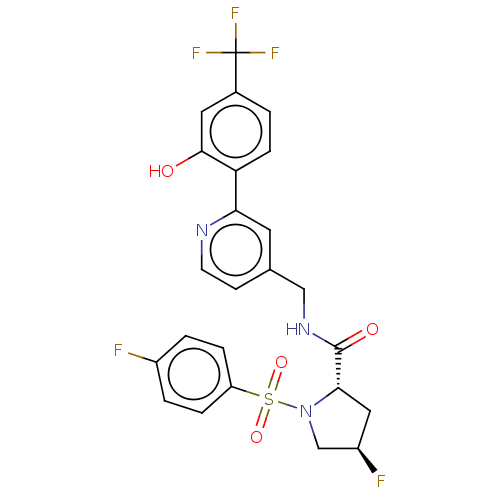
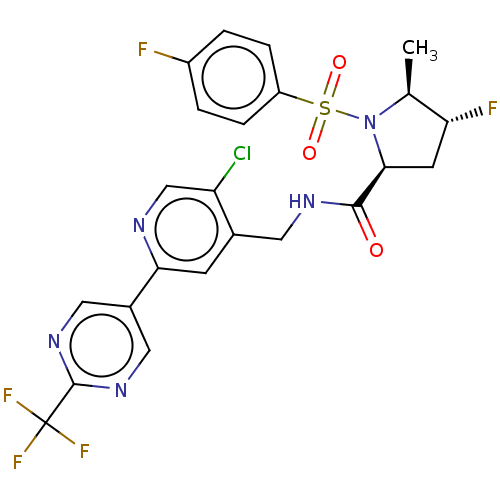
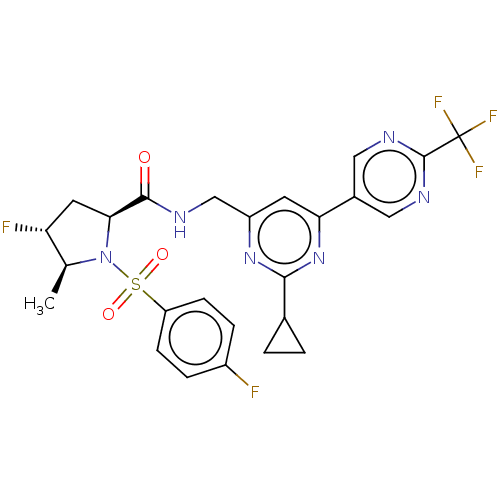
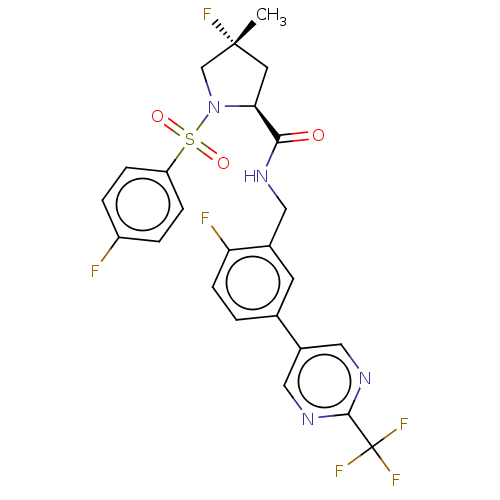
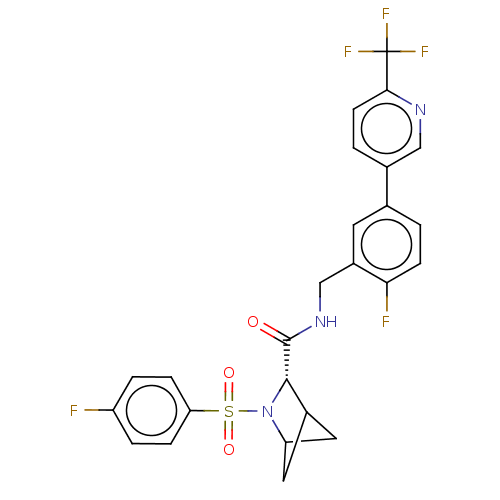
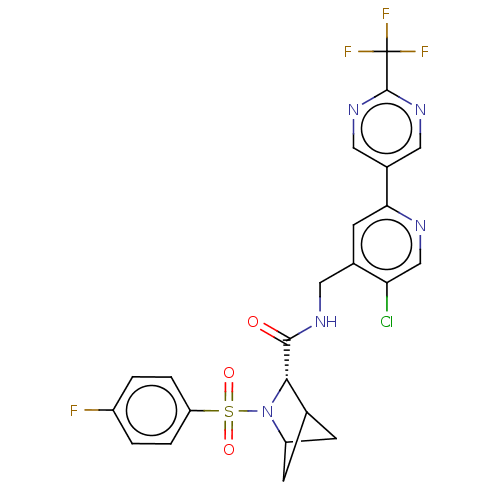
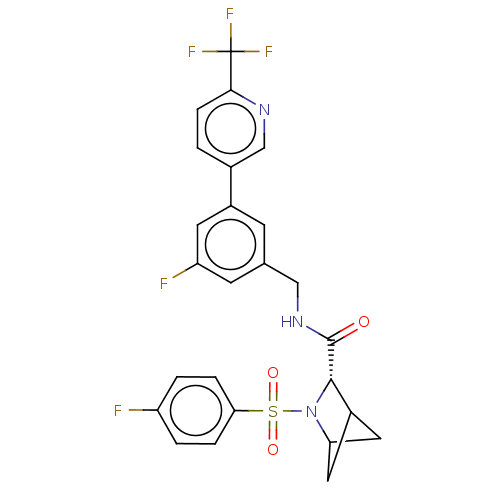
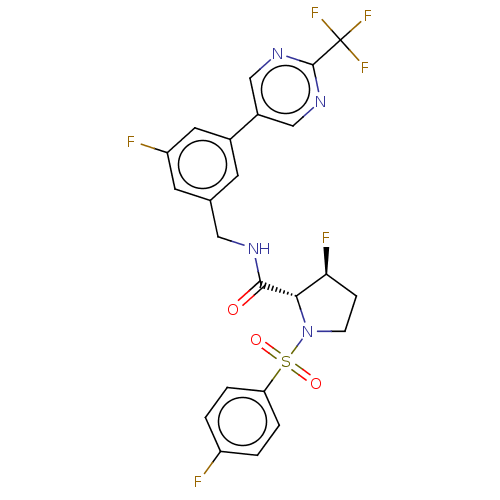
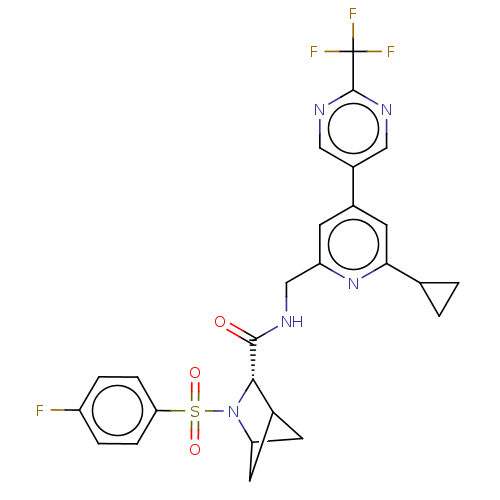
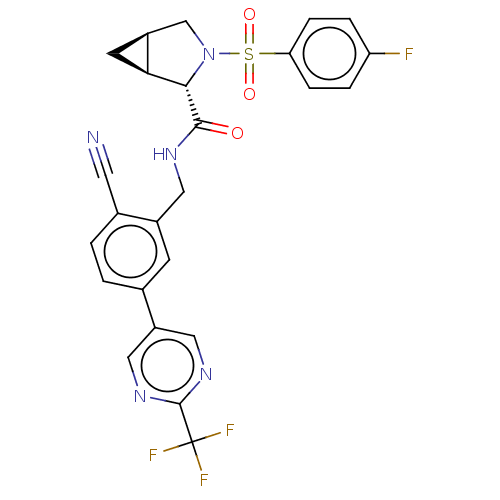
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 30nM ΔG°: -42.9kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
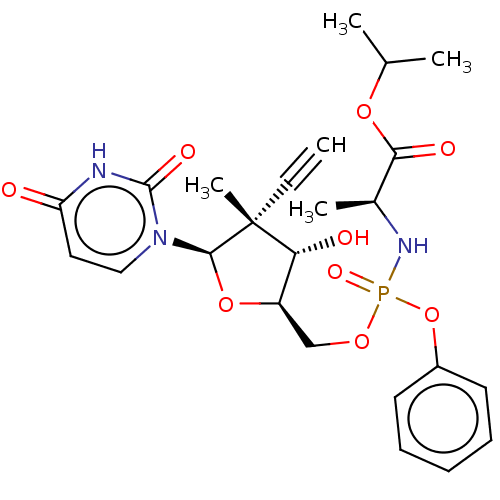
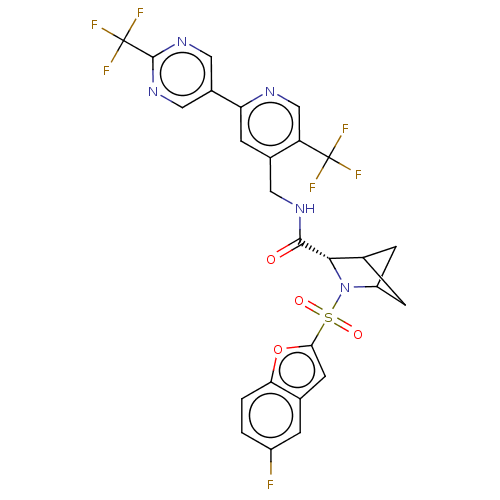
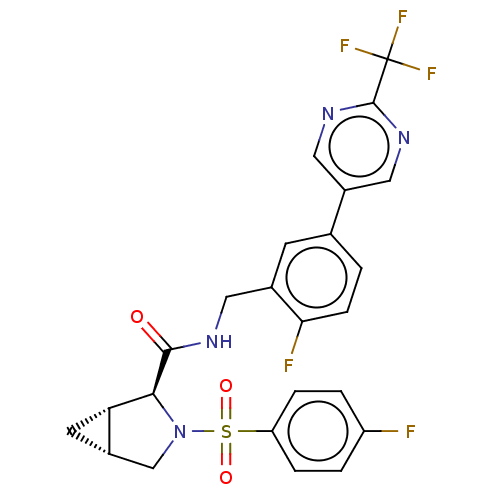
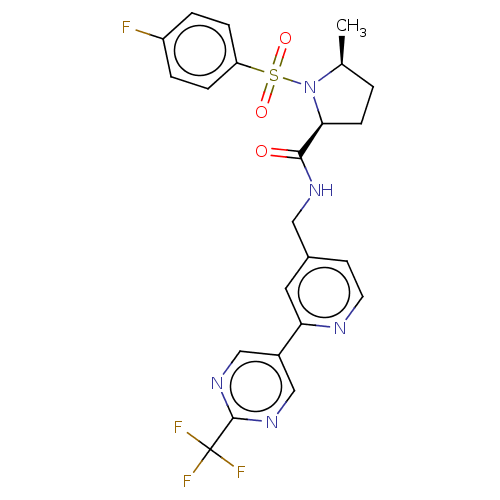
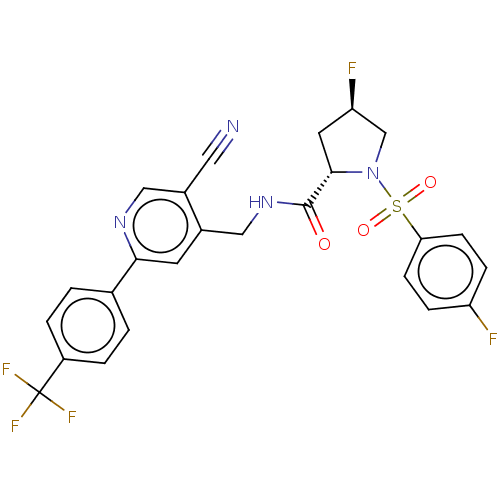
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 60nM ΔG°: -41.2kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
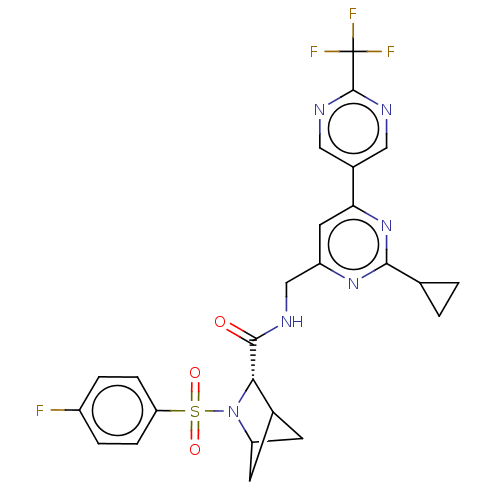
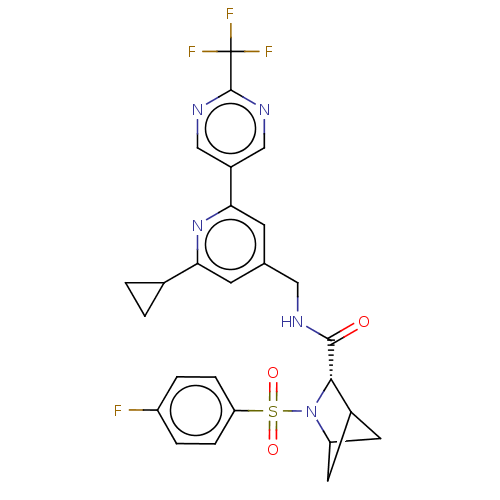
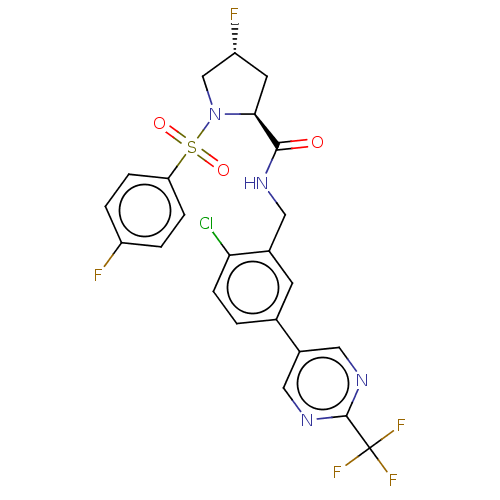
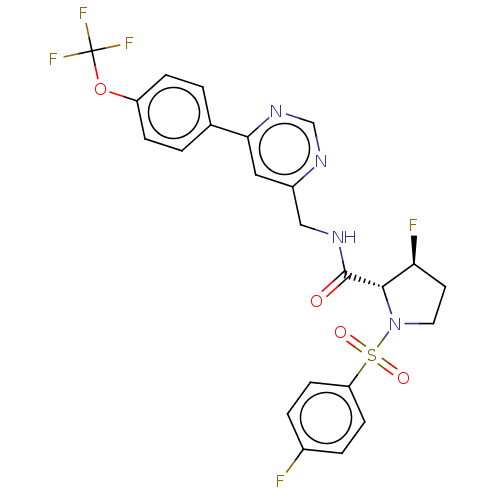
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 70nM ΔG°: -40.8kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
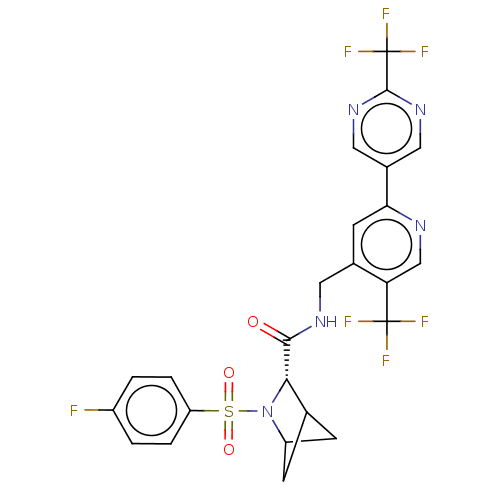
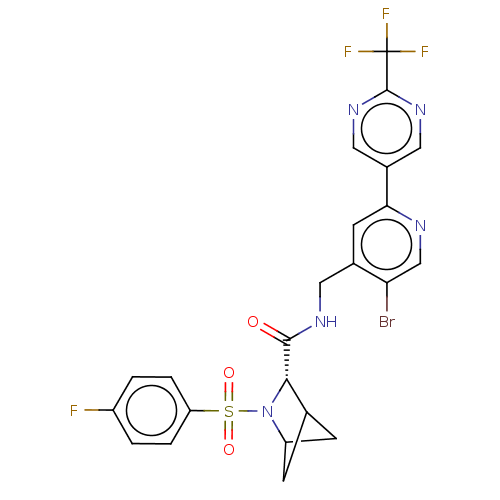
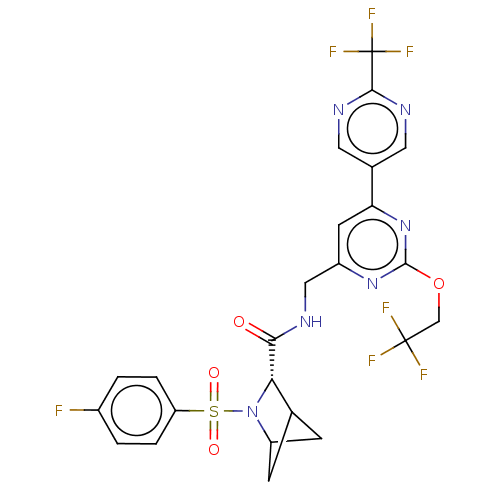
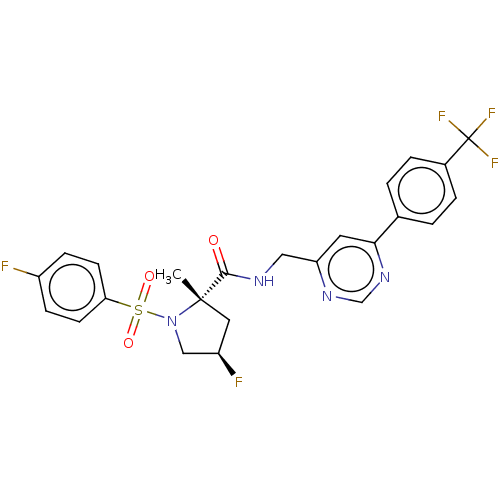
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 1.50E+3nM ΔG°: -33.2kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
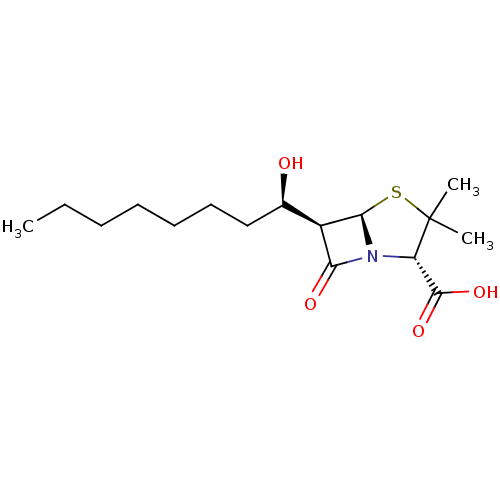
Affinity DataKi: 3.20E+3nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 4.70E+3nM ΔG°: -30.4kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 6.00E+3nM ΔG°: -29.8kJ/molepH: 7.3 T: 2°CAssay Description:To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ...More data for this Ligand-Target Pair
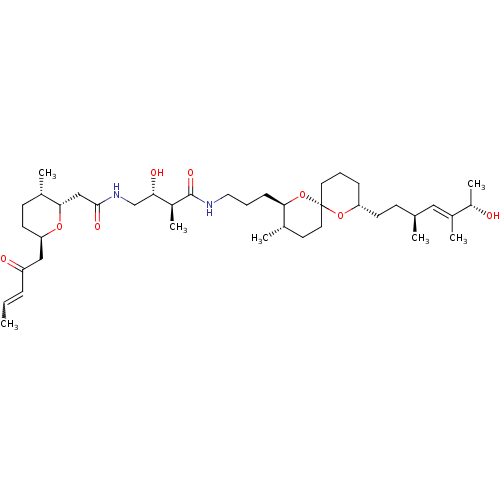
Affinity DataKi: 2.70E+4nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCdelta by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
Affinity DataKi: 4.20E+4nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
Affinity DataKi: 7.80E+4nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
Affinity DataKi: 8.30E+4nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
Affinity DataKi: 4.04E+5nMAssay Description:Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 1.08nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 1.18nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 1.55nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 1.85nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 2.15nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 2.61nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 2.77nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 3nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 3.55nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 3.82nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4.24nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4.64nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 4.84nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
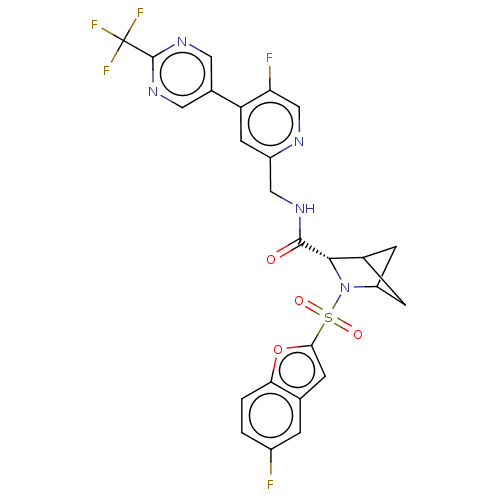
TargetSerine/threonine-protein kinase/endoribonuclease IRE1(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of IRE1 in human RPMI 8226 cells assessed as reduction in XBP1 splicing incubated for 3 hrs by RT-PCR methodMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5nMAssay Description:Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5nMAssay Description:Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5.20nMAssay Description:IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 5.20nMAssay Description:IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 6nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 6.06nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 6.08nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 6.11nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7.24nMAssay Description:IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7.88nMAssay Description:IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 7.88nMAssay Description:IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 8nMAssay Description:IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing...More data for this Ligand-Target Pair