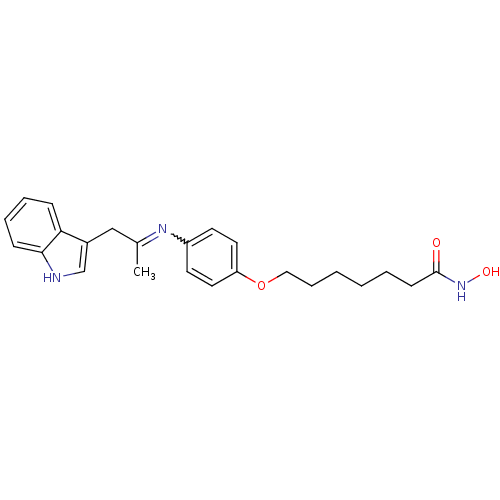
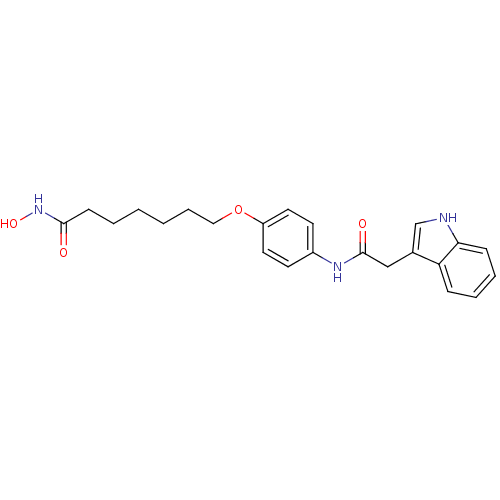
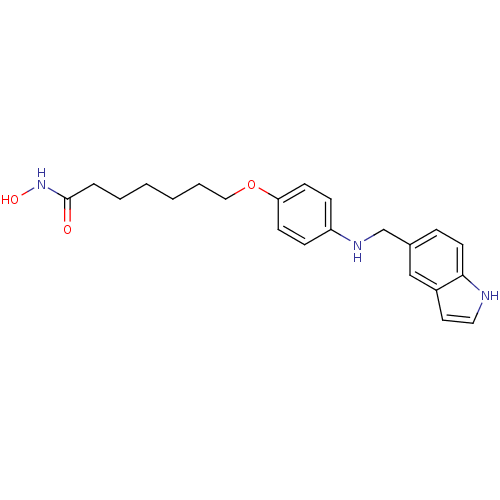
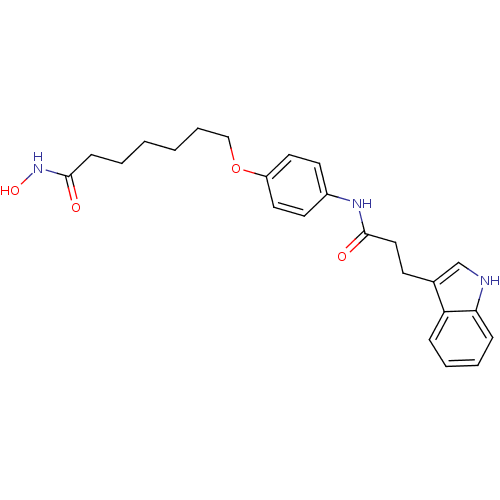
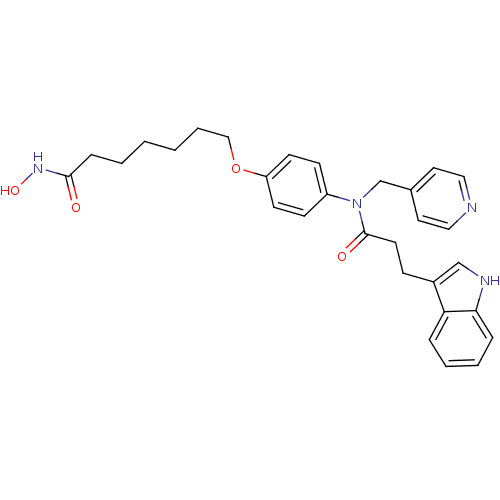
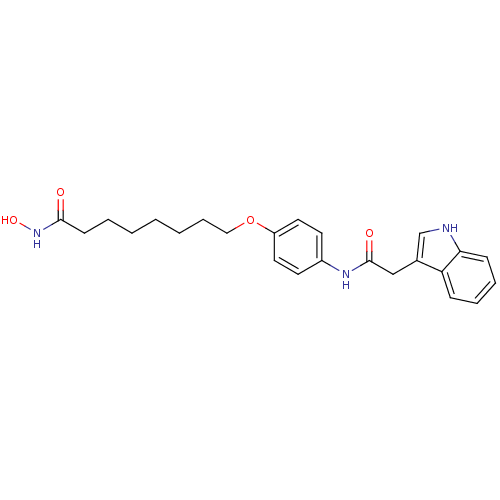
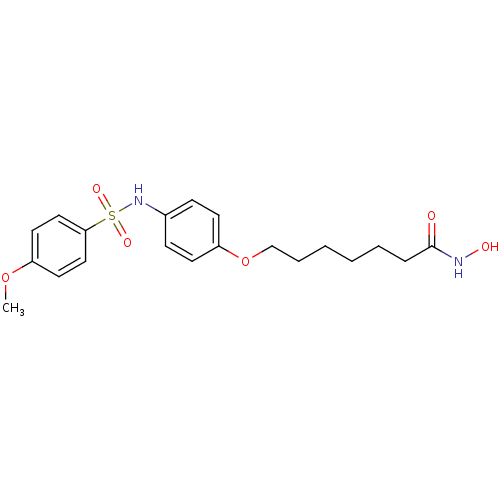
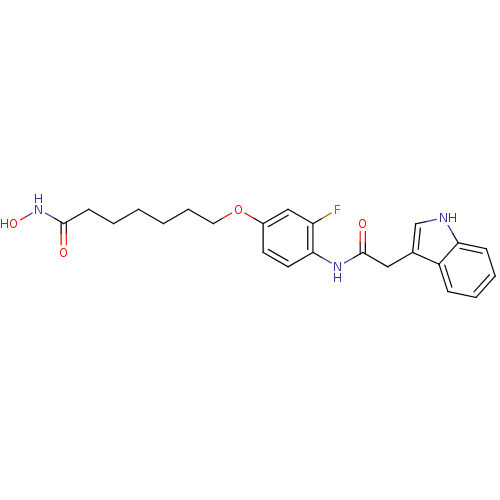
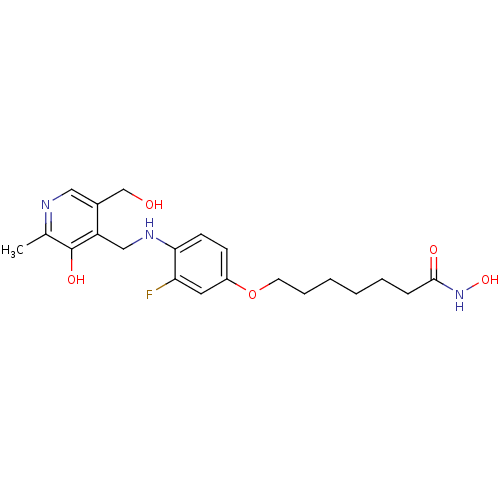
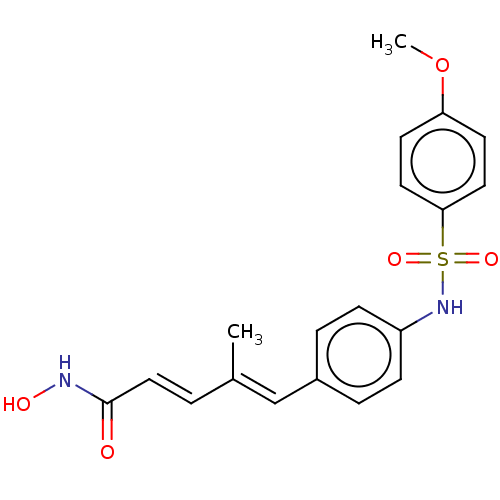
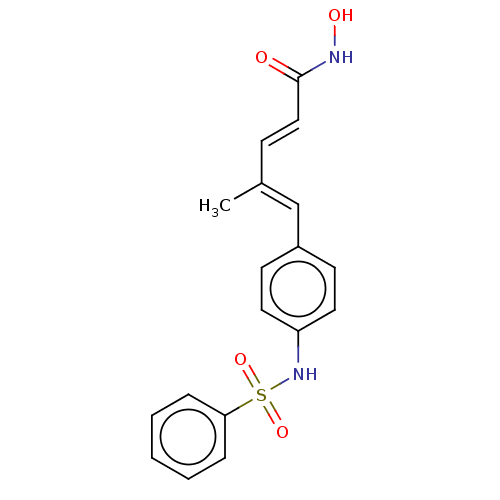
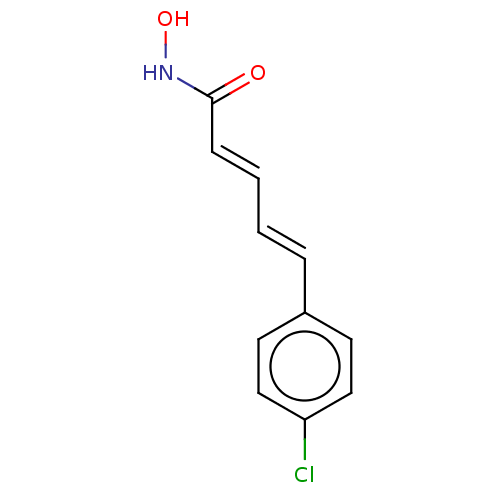
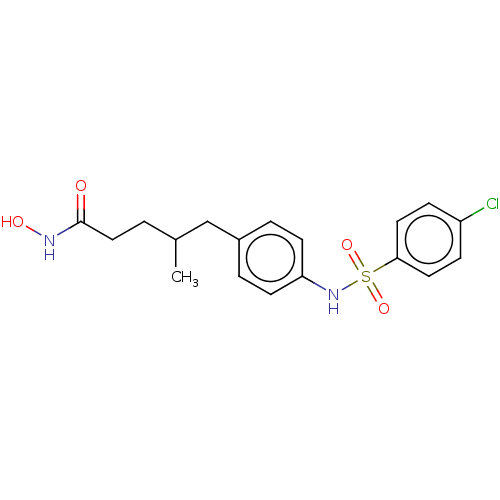
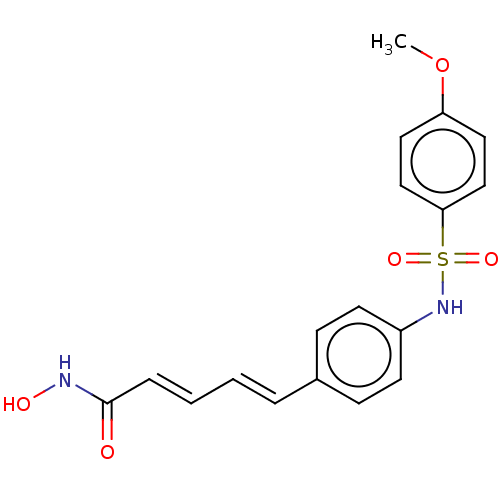
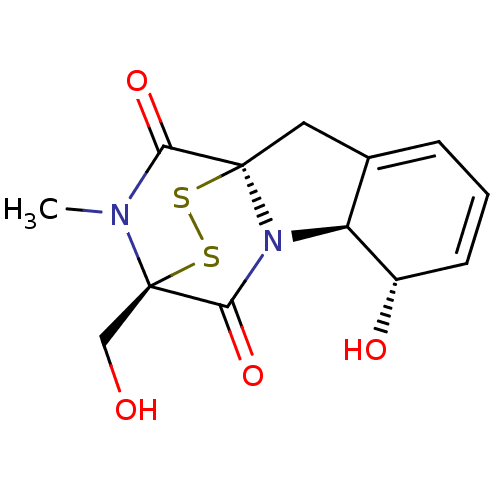
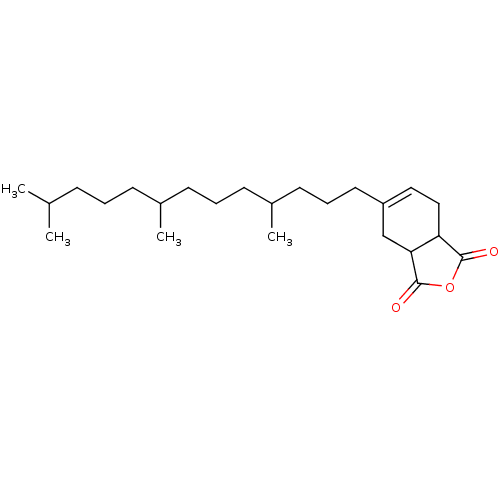
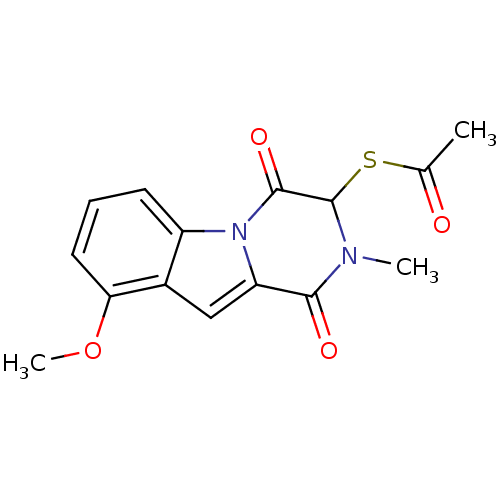
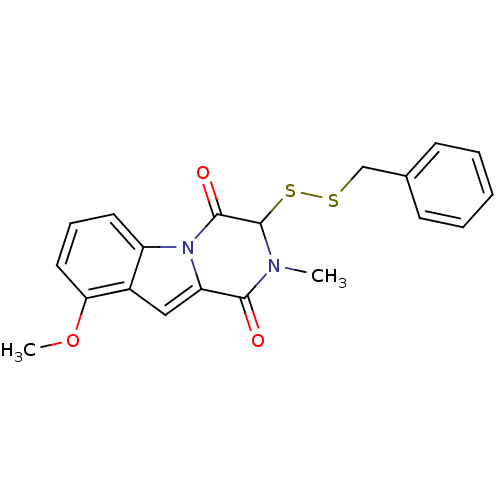
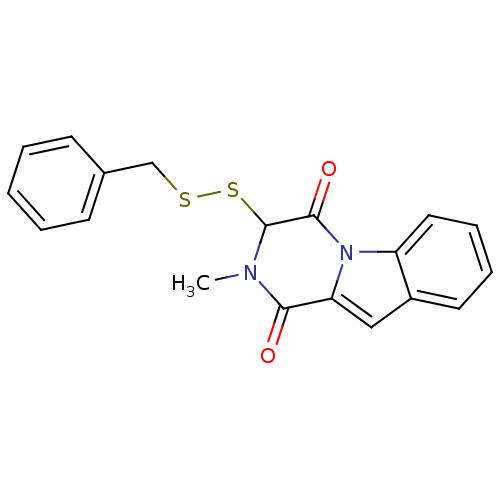
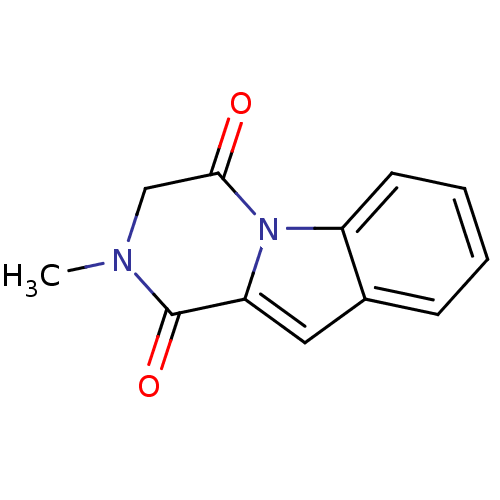
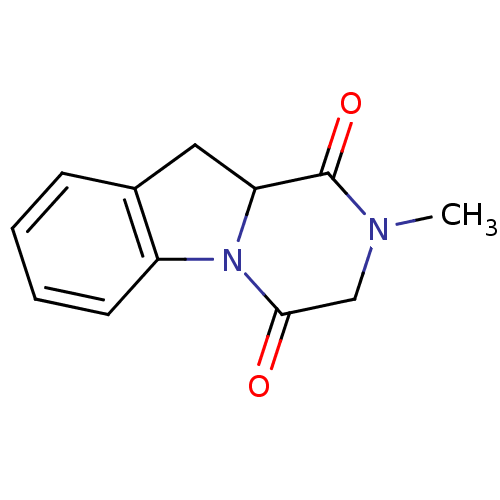
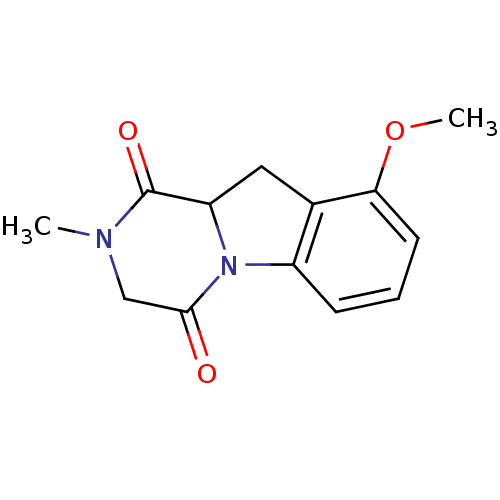
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme sourceMore data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 172nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 252nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 279nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 302nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 788nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+3nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 2.58E+3nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Binding affinity for human cloned Histamine H1 receptor expressed in CHO cells using [3H]pyrilamine as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 2.08E+4nMAssay Description:Binding affinity for human cloned 5-hydroxytryptamine 3 receptorMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 4.02E+4nMAssay Description:In vitro inhibition of geranylgeranyl-protein transferase type-IMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 9.03E+4nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: 9.20E+4nMAssay Description:In vitro inhibition of geranylgeranyl-protein transferase type-IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Imperial College
Curated by ChEMBL
Imperial College
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-GGPP incorporation into recombinant human Ha-Ras-CVLL by Geranylgeranyl transferase type IMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras-CVLS by farnesyl transferaseMore data for this Ligand-Target Pair

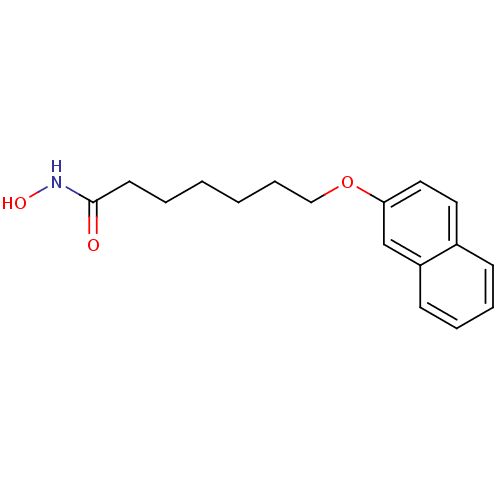
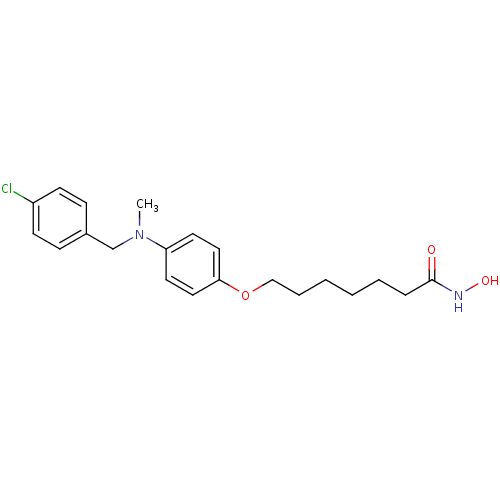

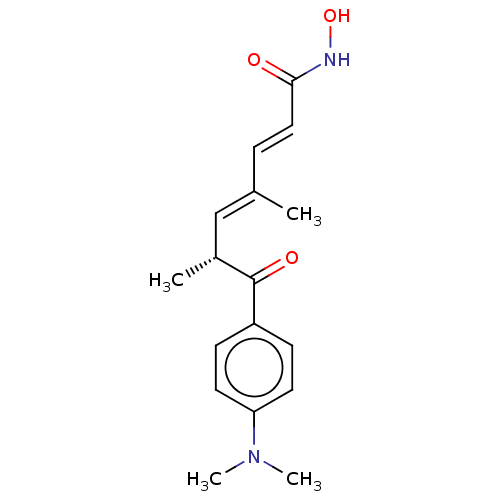
 3D Structure (crystal)
3D Structure (crystal)