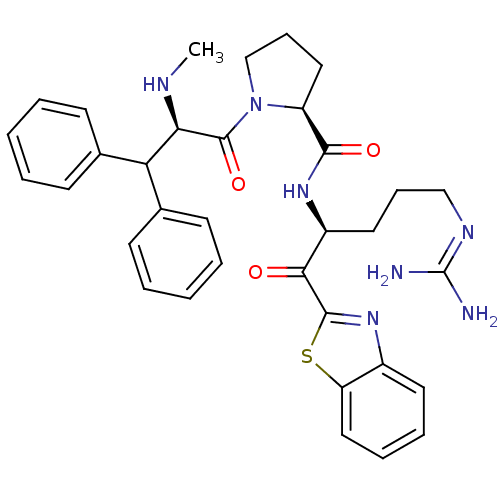
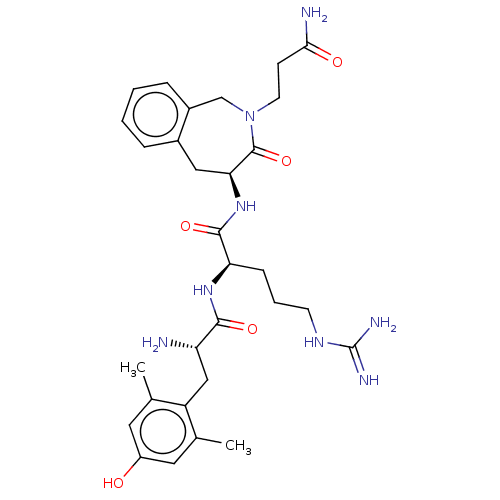
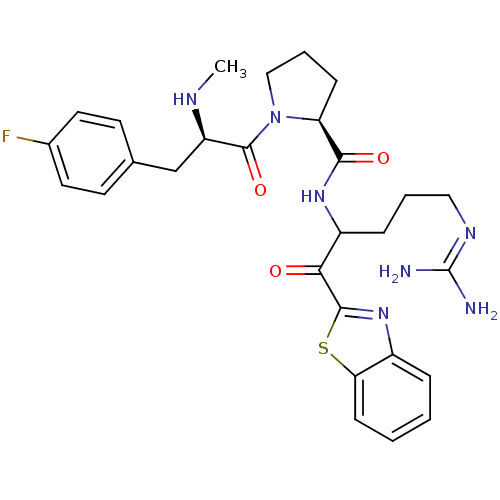
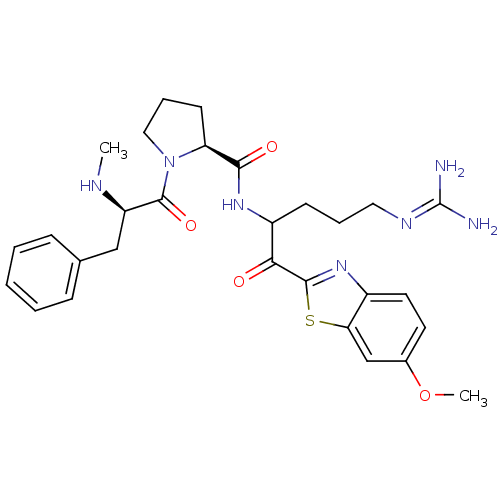
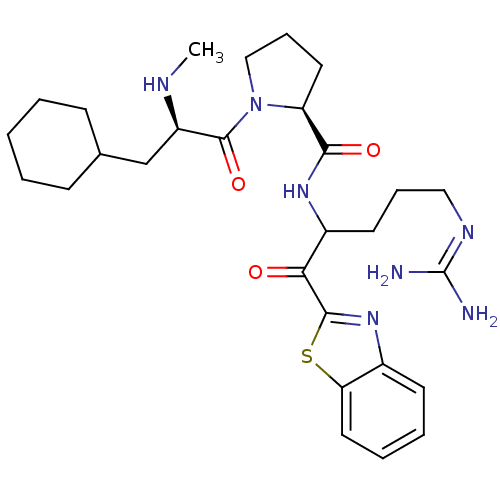
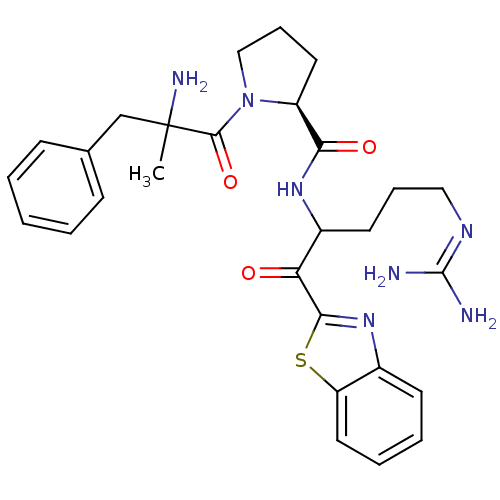
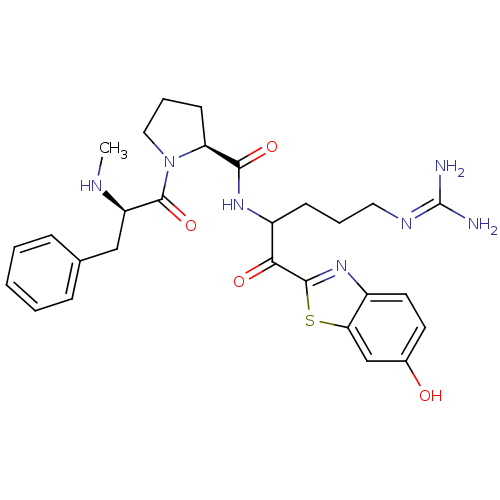
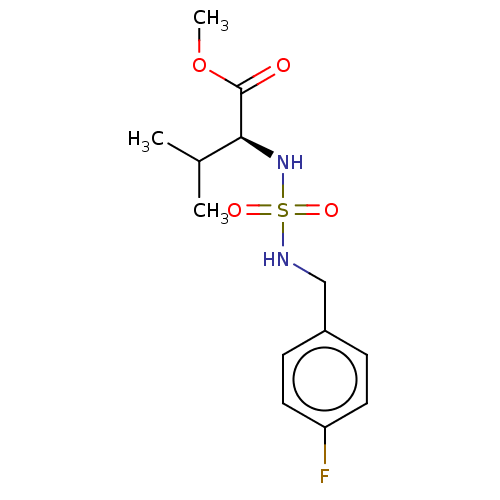
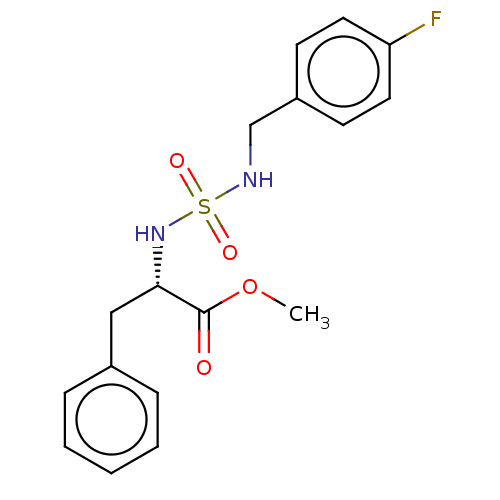
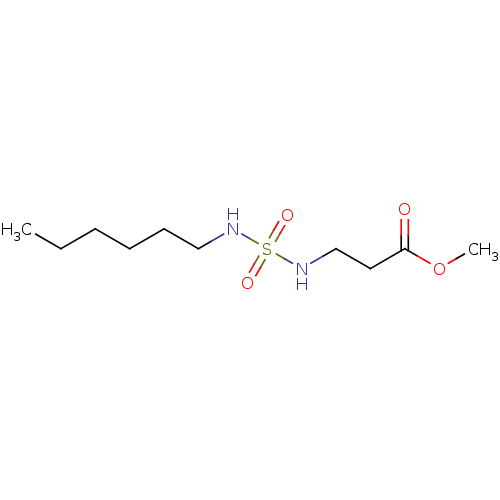
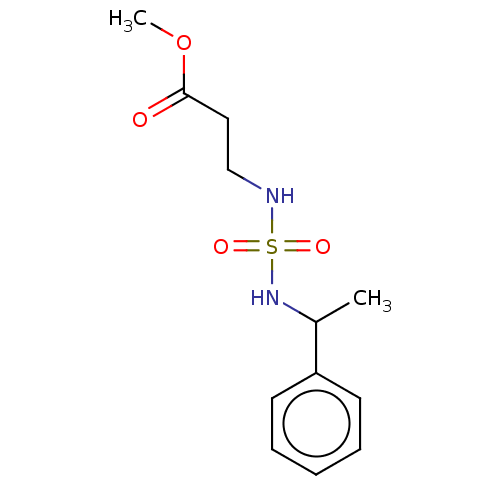
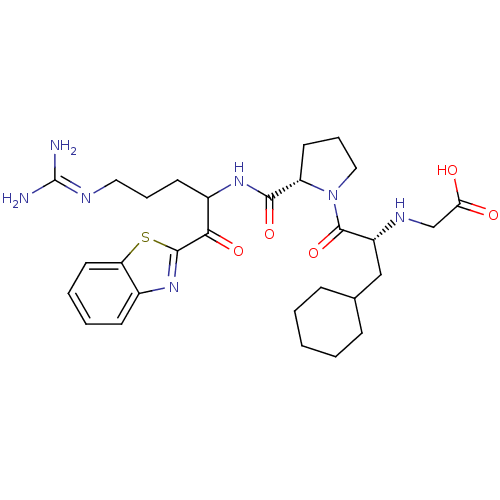
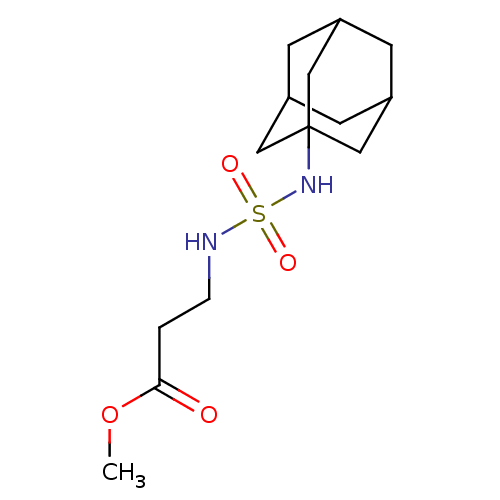
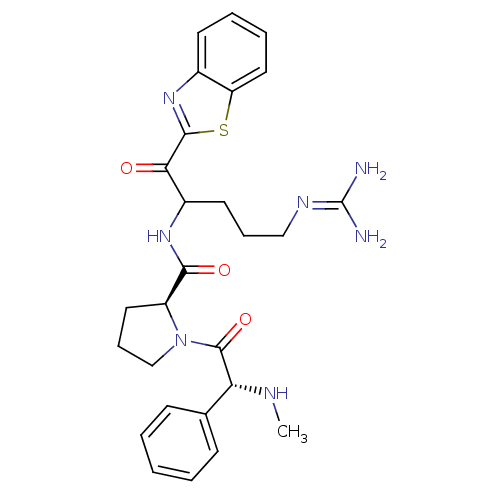
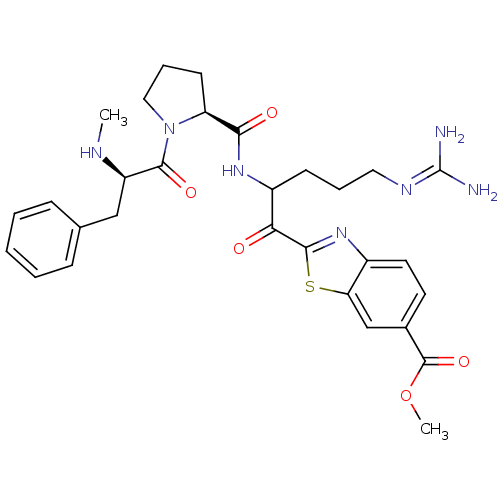
Affinity DataKi: 0.000650nM ΔG°: -72.4kJ/mole IC50: 4.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
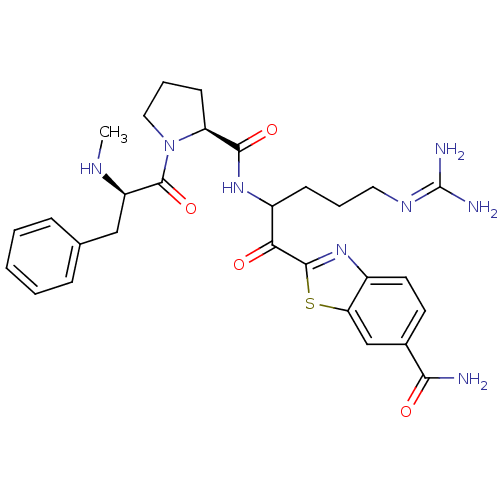
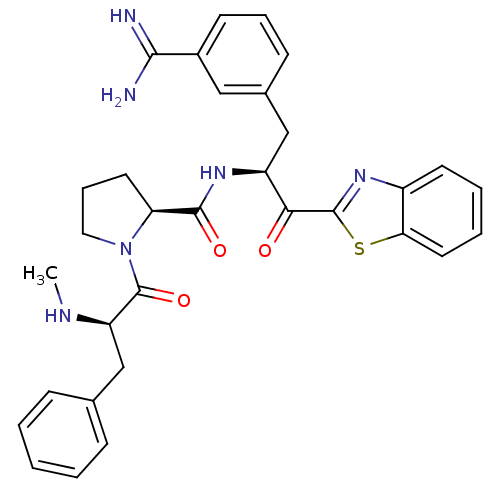
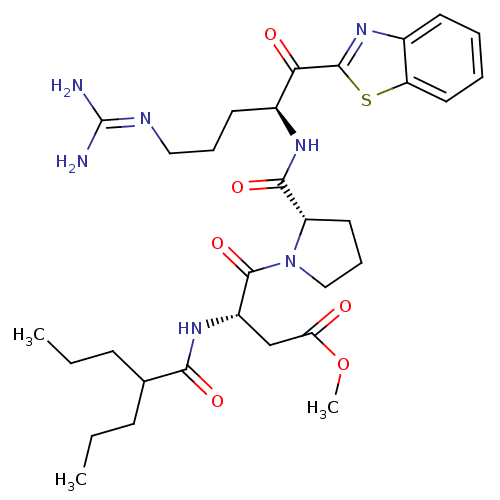
Affinity DataKi: 0.00550nM ΔG°: -66.9kJ/mole IC50: 21nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
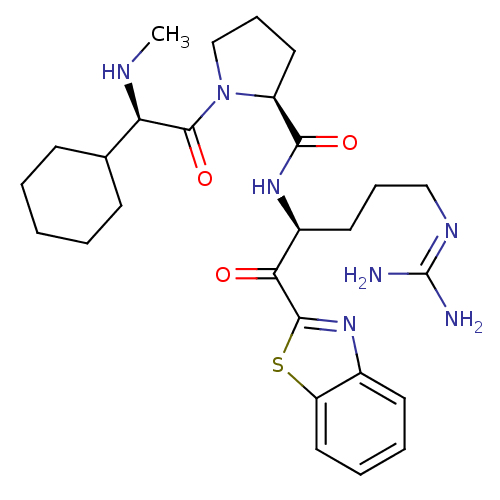
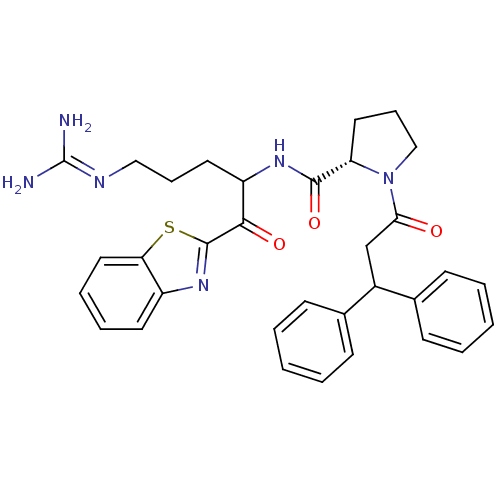
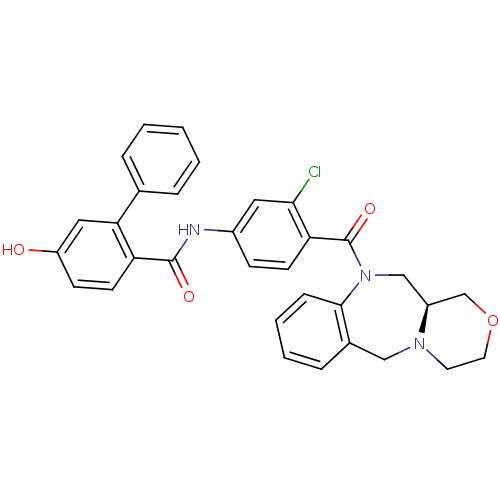
Affinity DataKi: 0.00700nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
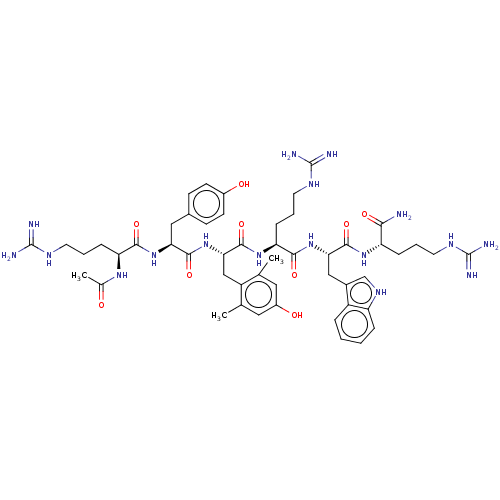
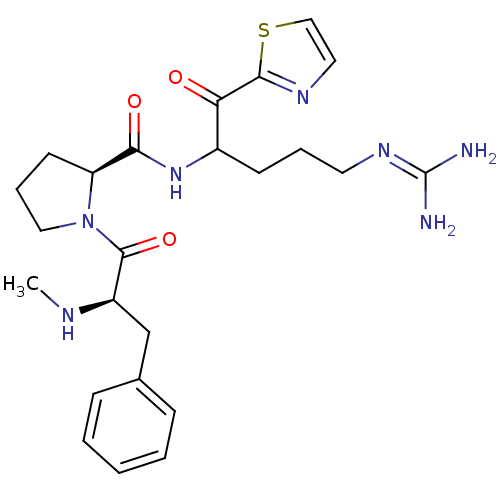
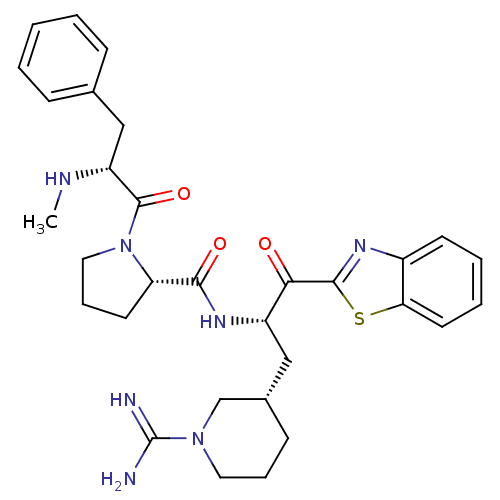
Affinity DataKi: 0.0180nM ΔG°: -63.8kJ/mole IC50: 5.30nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nMAssay Description:Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]diprenorphine from human mu opioid receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation counti...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -58.9kJ/mole IC50: 15nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM IC50: 7.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.180nM ΔG°: -57.9kJ/mole IC50: 48nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 3.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.230nM IC50: 3nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.340nM IC50: 38nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.360nM ΔG°: -56.1kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nM IC50: 7nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.450nMAssay Description:Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.460nM ΔG°: -55.4kJ/mole IC50: 34nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 0.580nM IC50: 60nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Inhibition of recombinant human carbonic anhydrase 12 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.740nMAssay Description:Inhibition of recombinant human carbonic anhydrase 12 preincubated for 15 mins by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.780nM IC50: 110nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -53.2kJ/mole IC50: 11nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nM IC50: 38nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM IC50: 56nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nM IC50: 95nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM IC50: 51nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair