Affinity DataKi: 38nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.62E+4nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.62E+4nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.43E+4nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.43E+4nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.48E+4nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.39E+4nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 9.60E+4nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 9.60E+4nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 9.90E+4nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+5nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.49E+5nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.49E+5nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.86E+5nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.92E+5nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.92E+5nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.54E+5nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.61E+5nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.61E+5nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.74E+5nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.77E+5nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataKi: 6.18E+5nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.72E+4nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.45E+4nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.69E+5nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+5nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.41E+5nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.43E+5nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.45E+5nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 330nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 65nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 440nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 6.5nMAssay Description:Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter...More data for this Ligand-Target Pair
Affinity DataEC50: 60nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 170nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.610nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMAssay Description:Activity at human ERbeta expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMAssay Description:Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g...More data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMAssay Description:Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g...More data for this Ligand-Target Pair
Affinity DataEC50: 0.850nMAssay Description:Agonist activity at ERbeta expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter g...More data for this Ligand-Target Pair

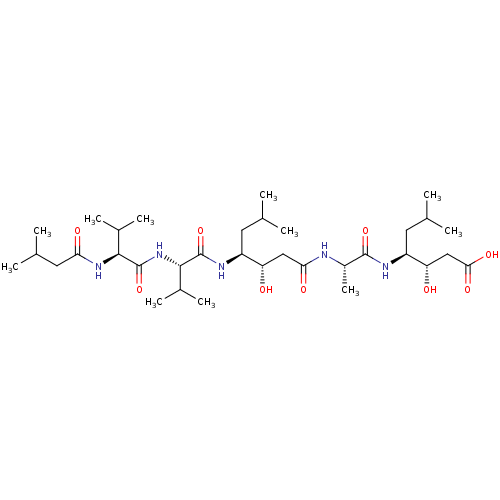
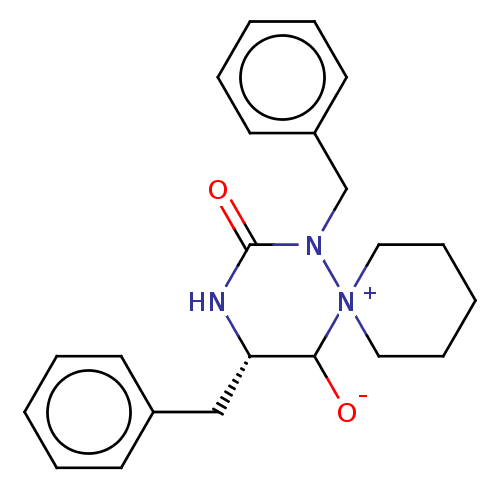

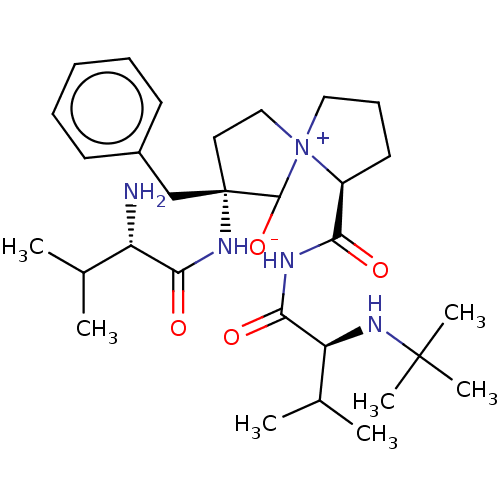
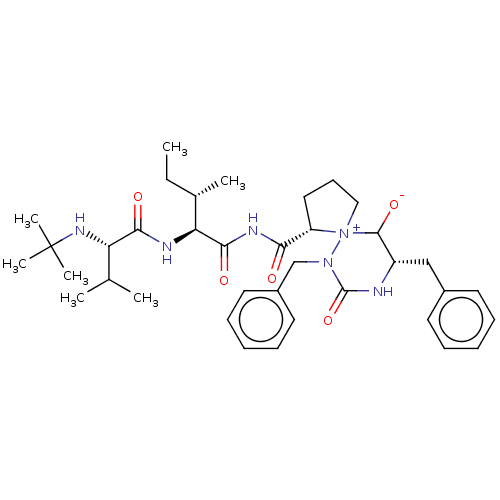

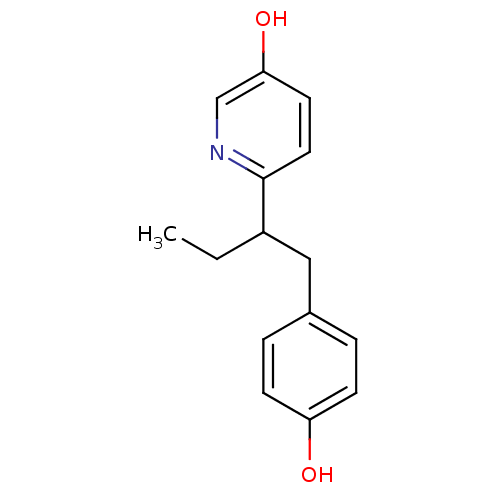

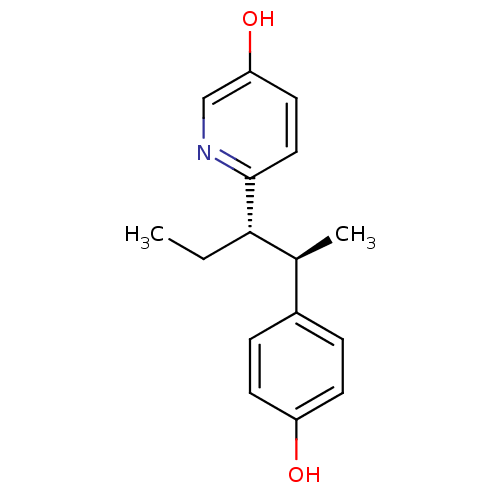


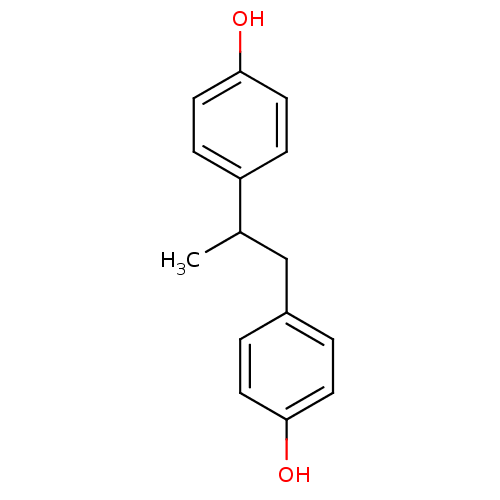
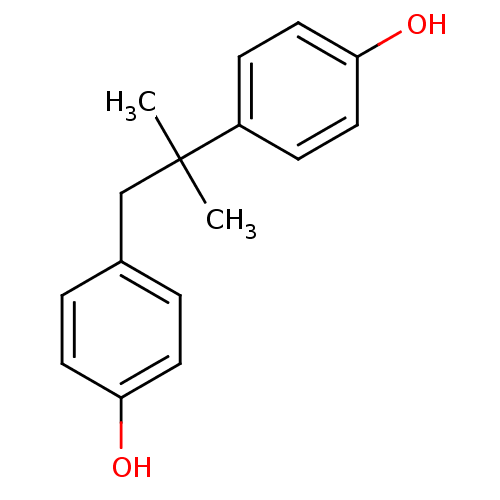
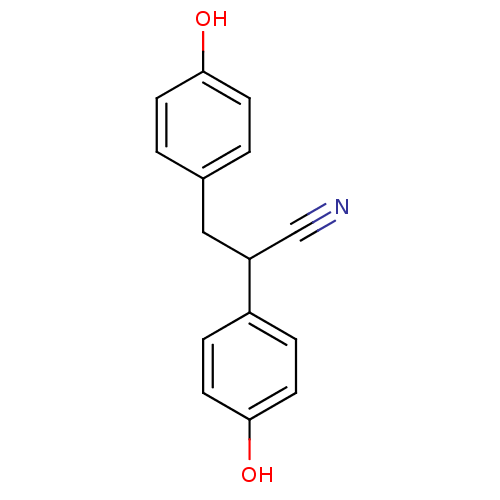
 3D Structure (crystal)
3D Structure (crystal)