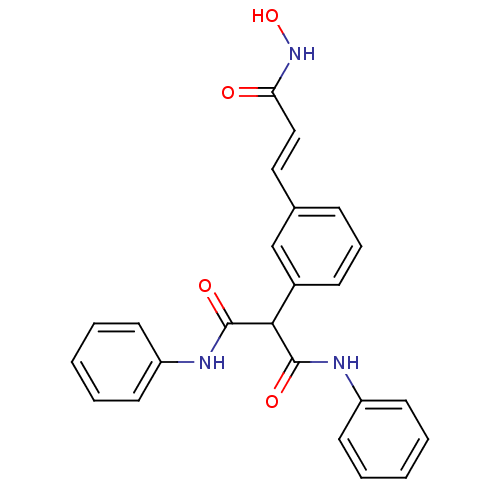
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against human Histone deacetylase 1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against human Histone deacetylase 1More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibitory activity against human Histone deacetylase 1More data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
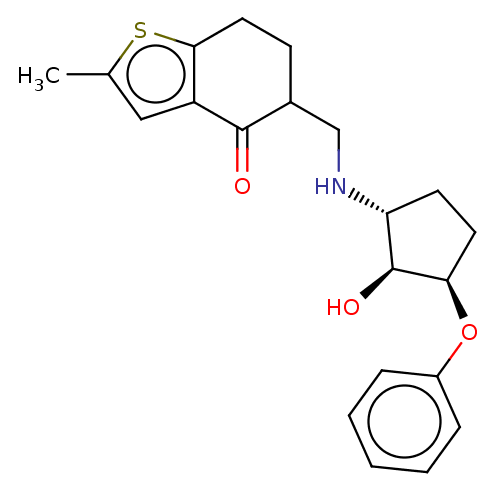
Affinity DataIC50: 8nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
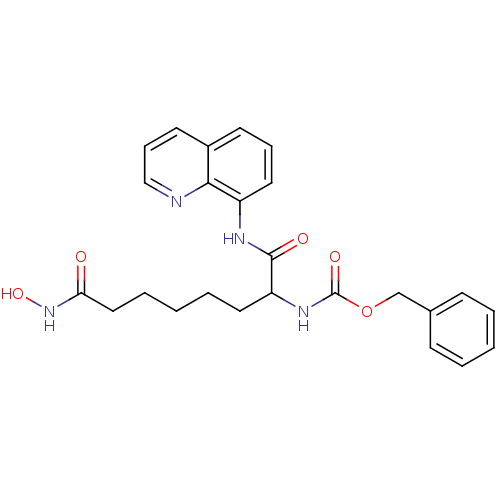
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against maize Histone deacetylase 2More data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibitory activity against human Histone deacetylase 1More data for this Ligand-Target Pair
Affinity DataIC50: 513nMAssay Description:Inhibitory activity against mouse Histone deacetylase 1 (HDAC1)More data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair


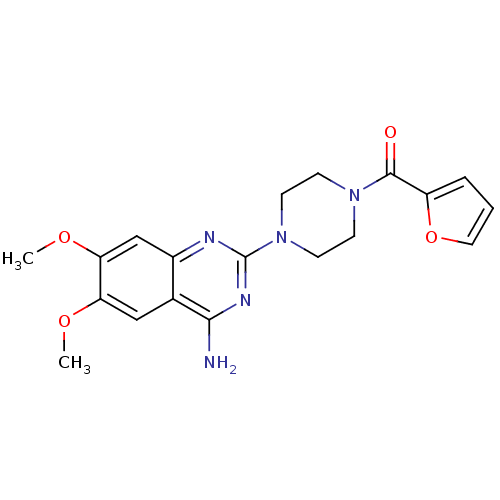


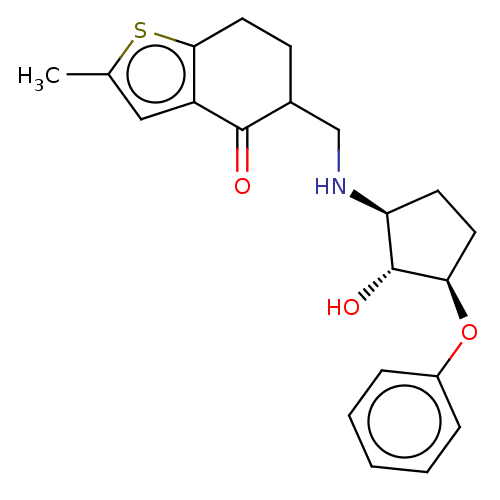
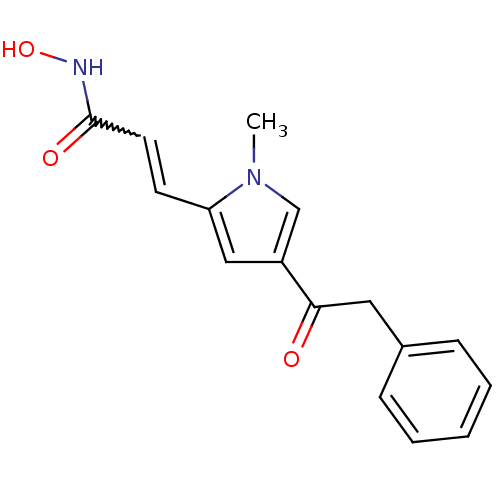
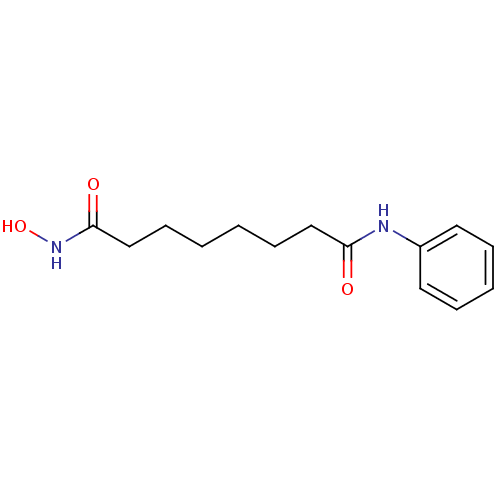
 3D Structure (crystal)
3D Structure (crystal)