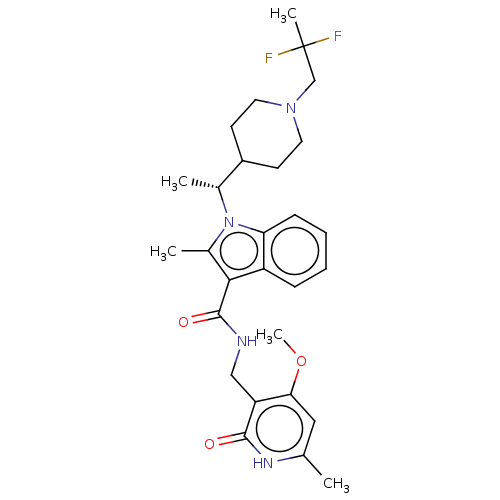
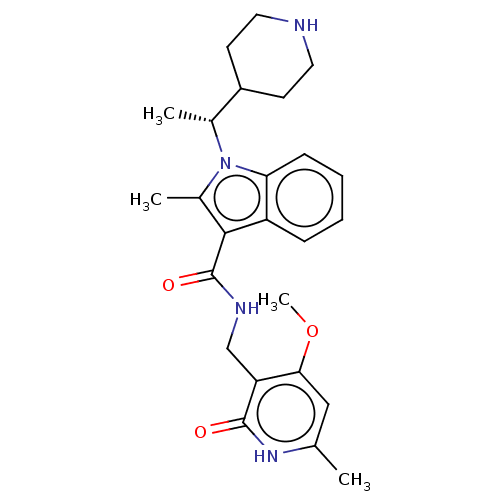
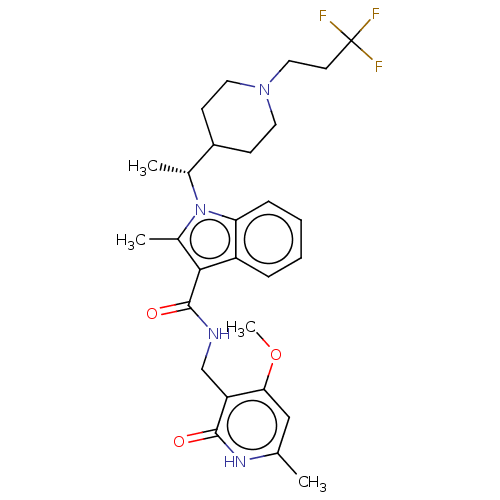
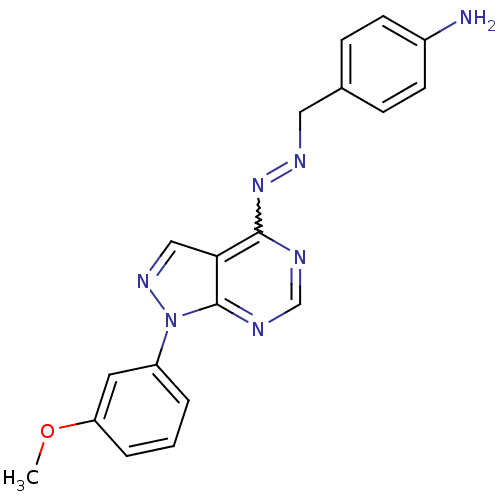
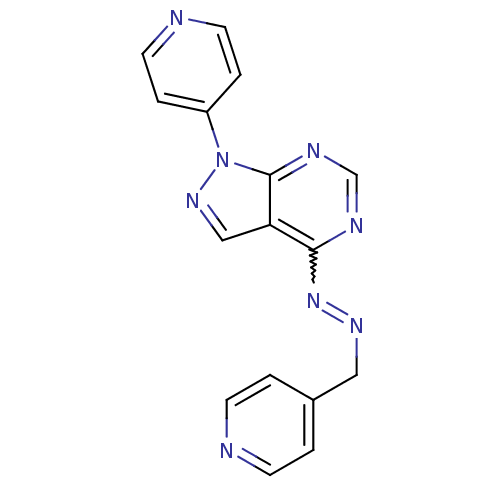
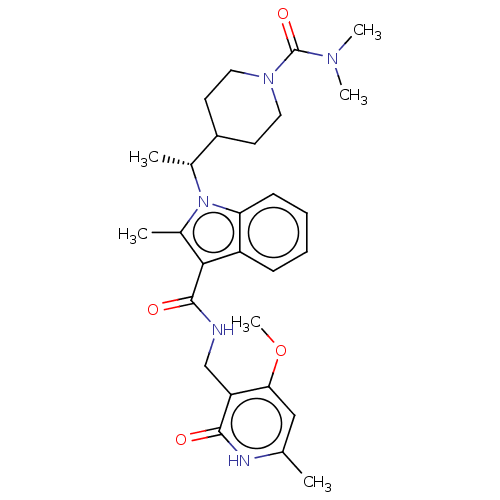
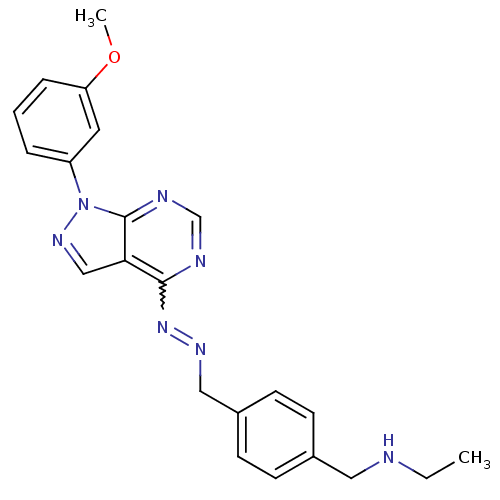
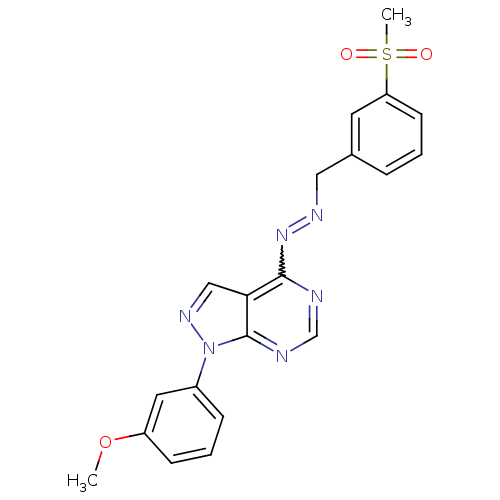
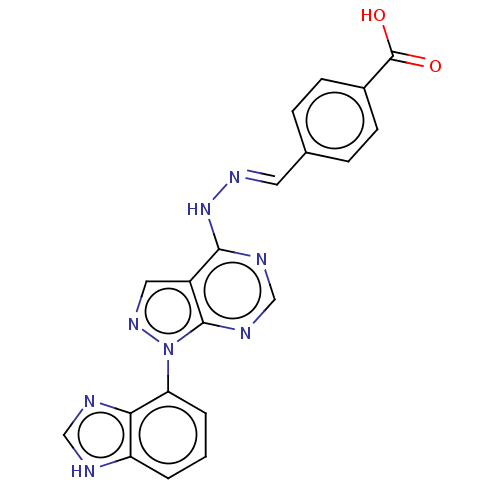
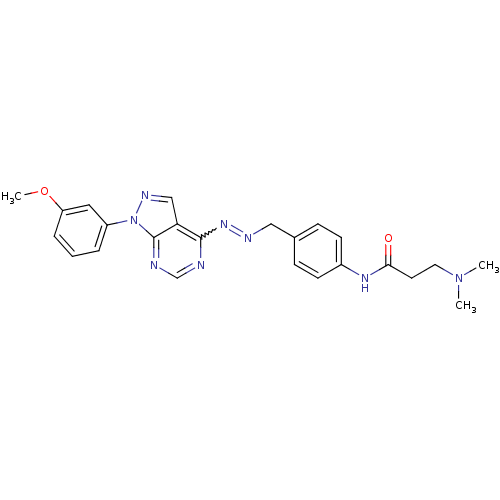
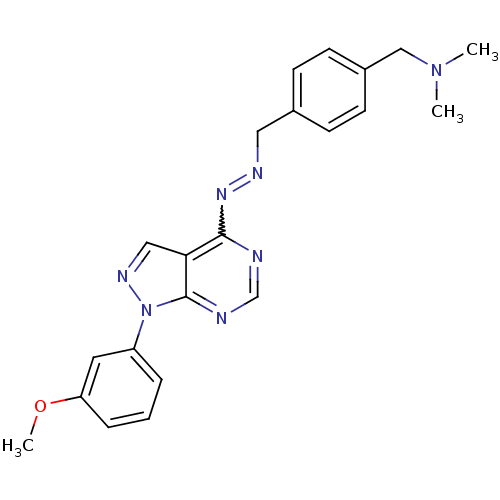
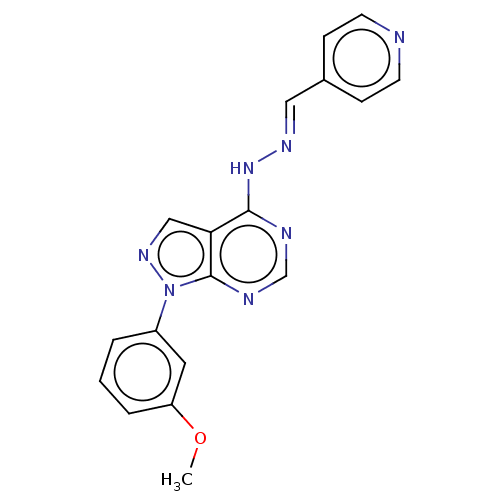
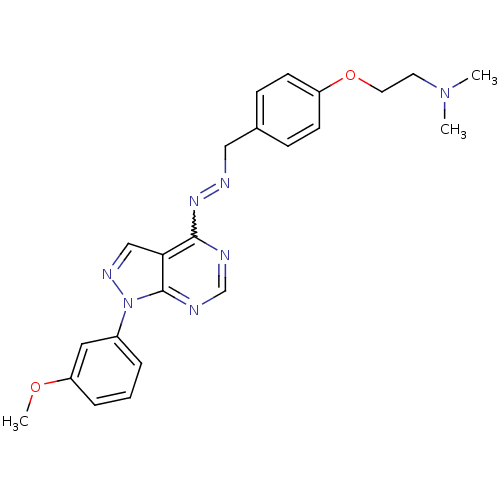
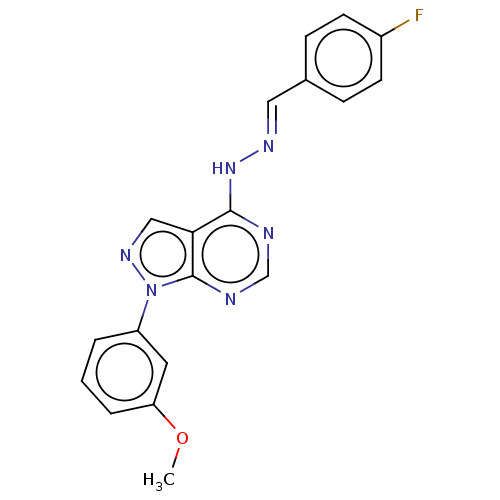
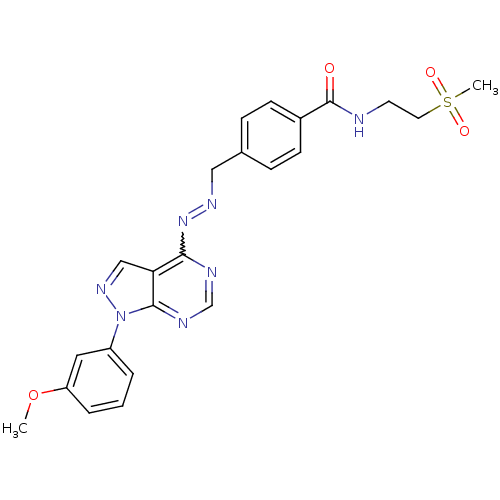
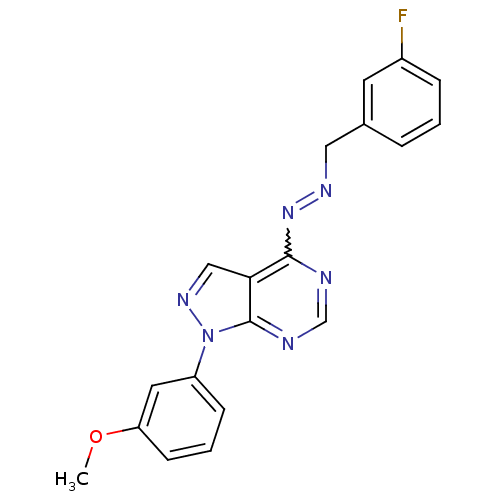
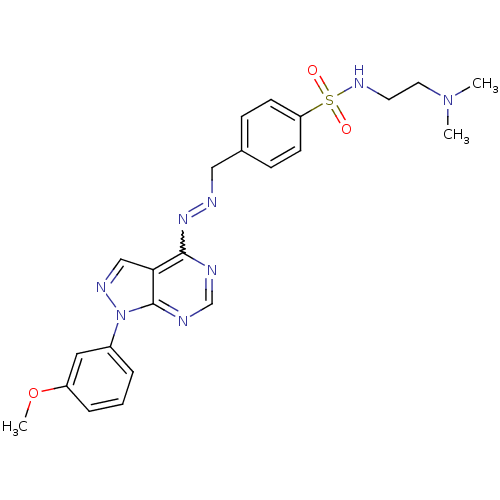
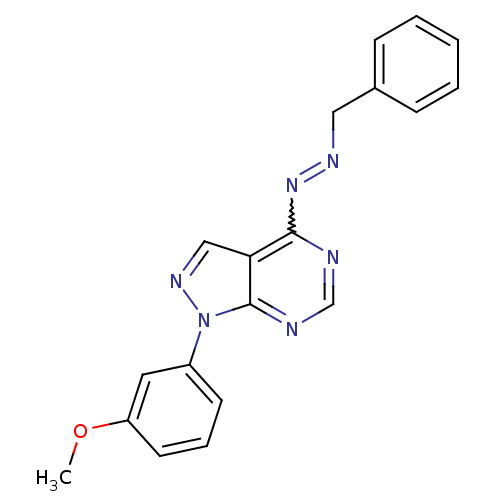
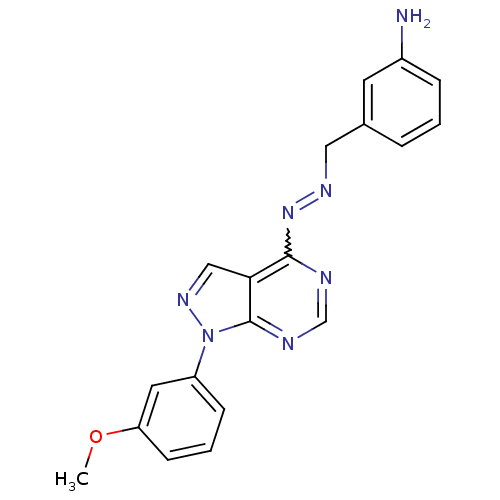
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.10nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 5.01nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 8.90nMAssay Description:Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 12.6nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair