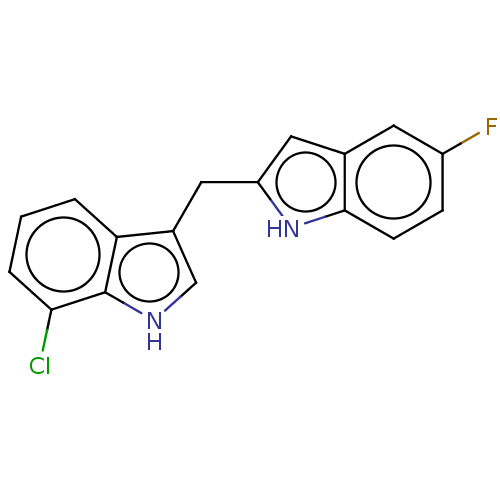
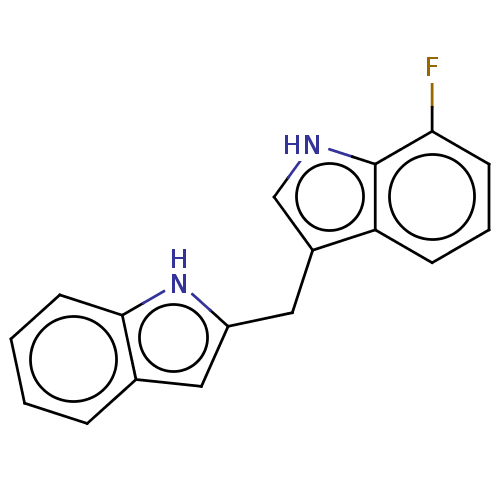
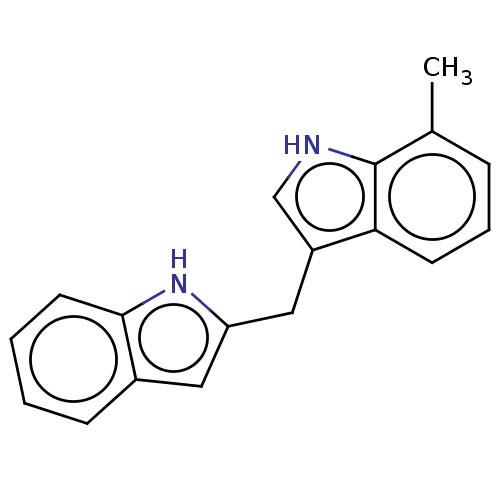
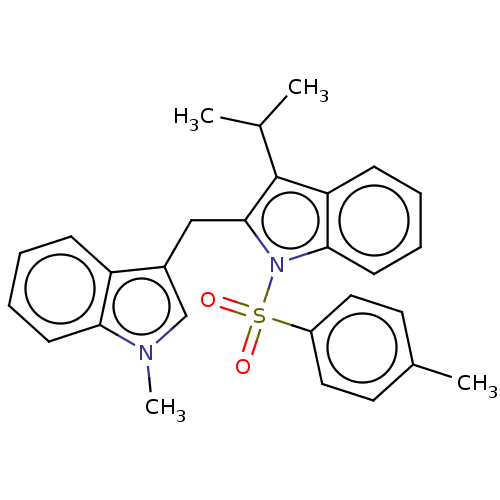
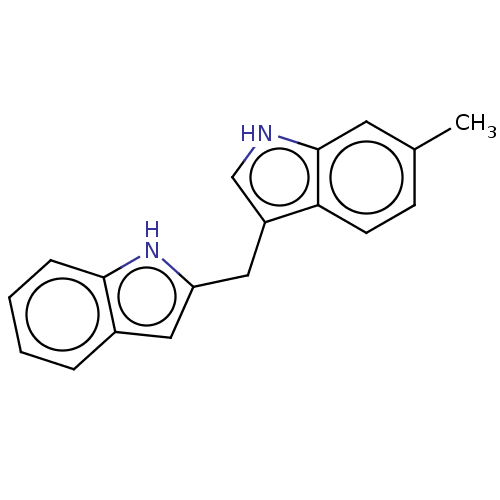
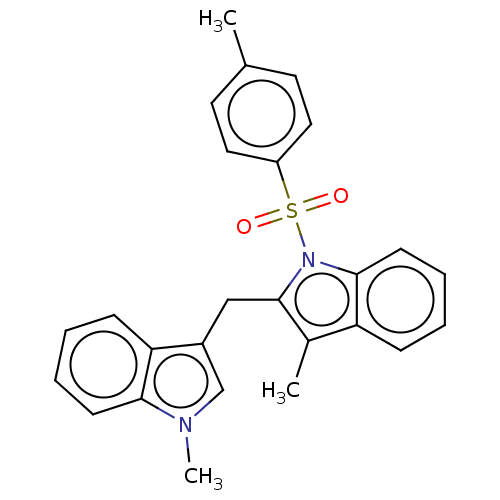
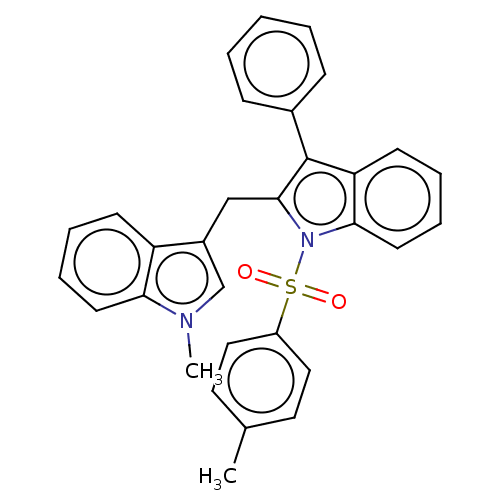
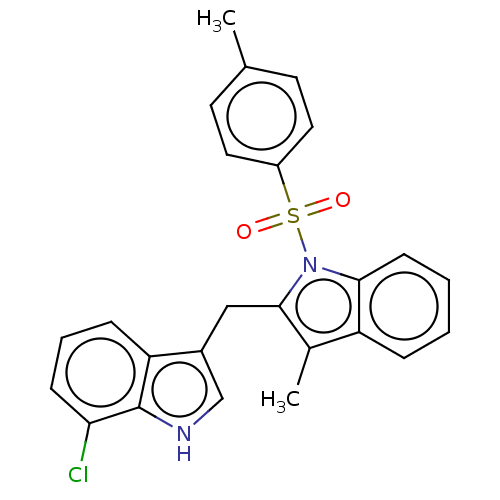
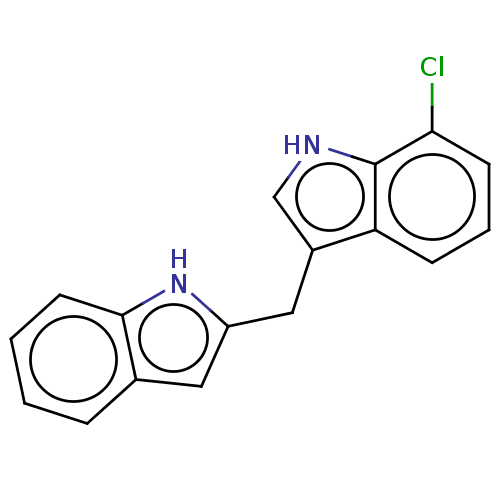
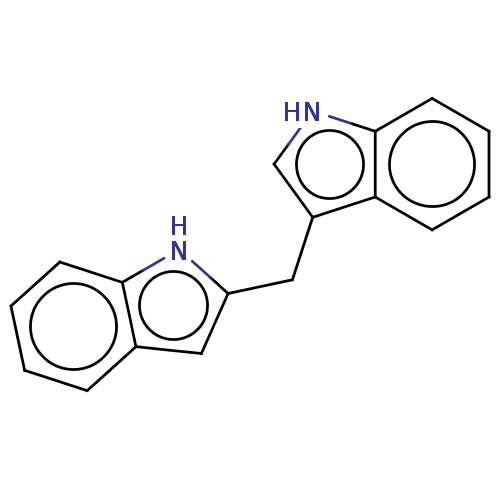
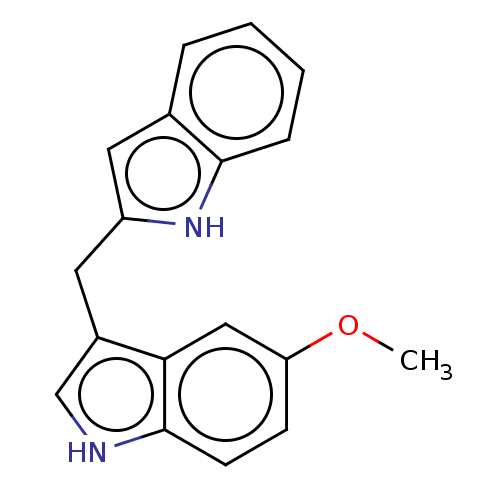
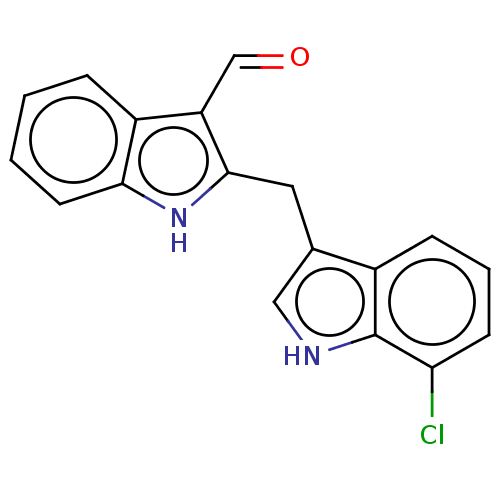
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
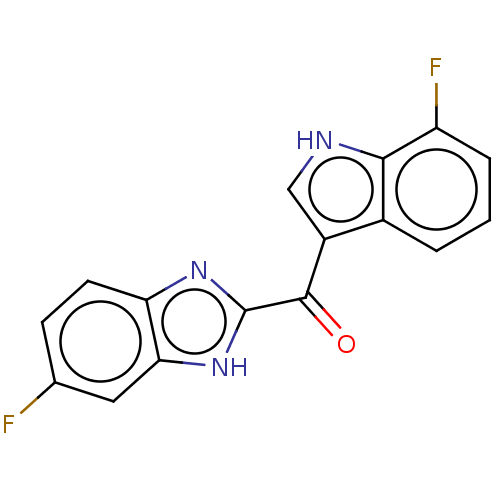
Affinity DataIC50: 202nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 24 hrs followed by medium replenishment and measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
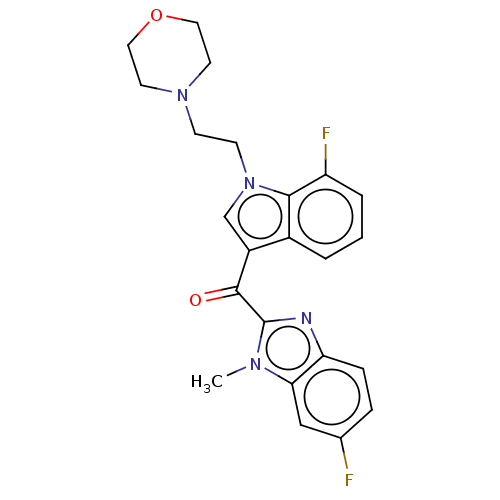
Affinity DataIC50: 250nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
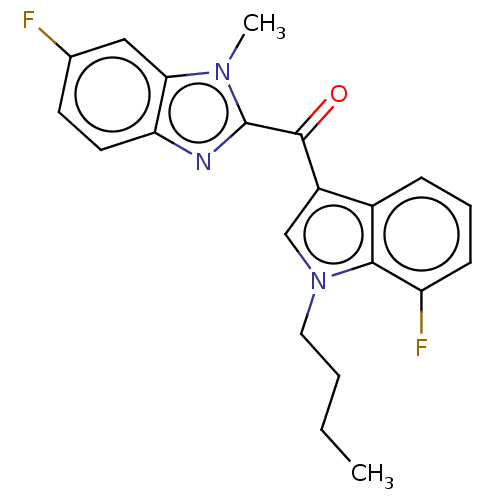
Affinity DataIC50: 300nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 230nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 215nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: >6.70E+3nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 94nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 86nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 270nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 93nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 230nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 215nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: >6.70E+3nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 94nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 86nMAssay Description:This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C....More data for this Ligand-Target Pair
Affinity DataEC50: 0.260nMAssay Description:Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 348nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 2.5nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 0.150nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 1.20nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 11nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 40nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 88nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 0.280nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 0.690nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 93nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 270nMAssay Description:AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles.More data for this Ligand-Target Pair
Affinity DataEC50: 270nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: 93nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: 230nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: 215nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
Affinity DataEC50: >6.70E+3nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair
TargetProprotein convertase subtilisin/kexin type 9(Homo sapiens (Human))
University Of Wisconsin
Curated by ChEMBL
University Of Wisconsin
Curated by ChEMBL
Affinity DataEC50: 161nMAssay Description:Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun...More data for this Ligand-Target Pair
Affinity DataEC50: 86nMAssay Description:Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assayMore data for this Ligand-Target Pair