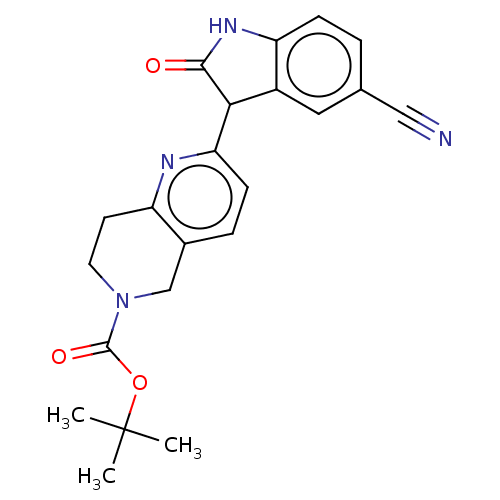
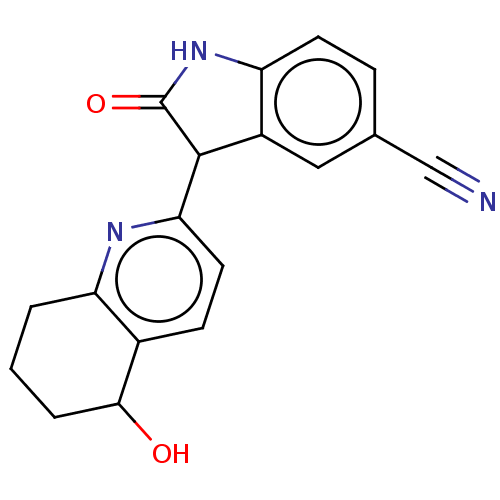
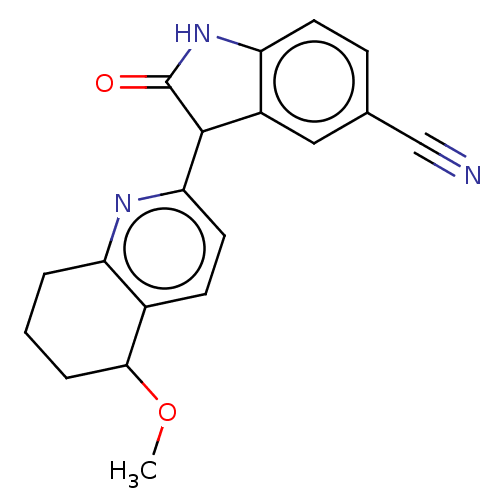
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:Compounds were tested for their ability to inhibit human Glycogen Synthase Kinase-3 beta (hGSK-3β) to phosphorylate biotin-YRRAAVPPSPSLSRHSSPHQ(...More data for this Ligand-Target Pair
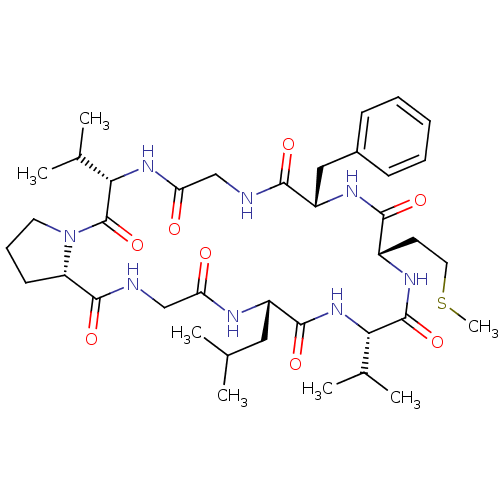
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibition of plasminMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30E+4nMAssay Description:Inhibition of human recombinant caspase 8 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of bovine Cathepsin BMore data for this Ligand-Target Pair