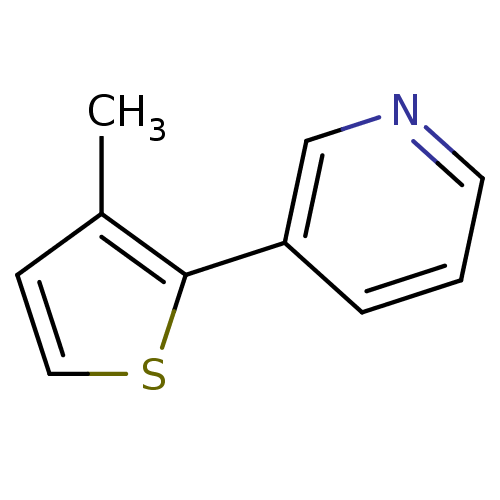
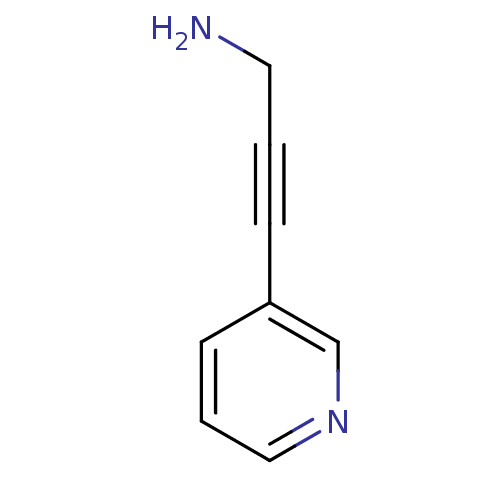
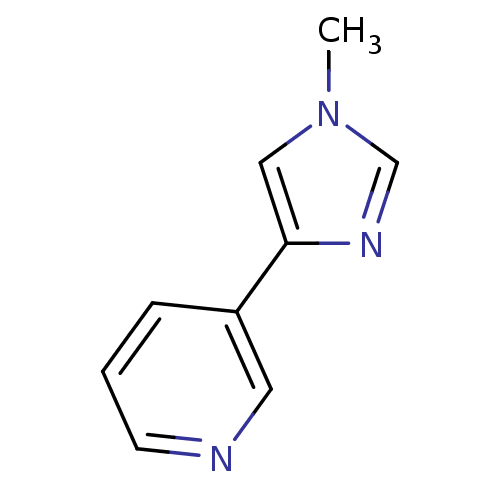
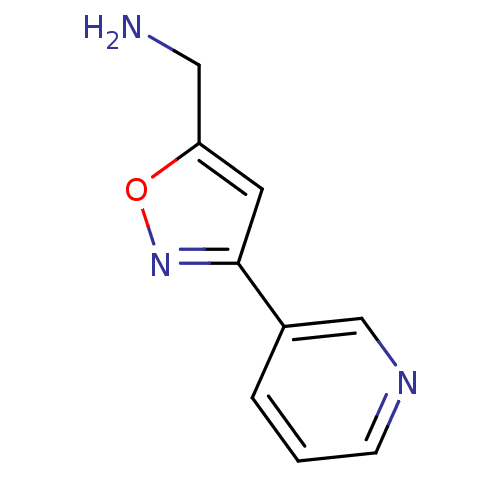
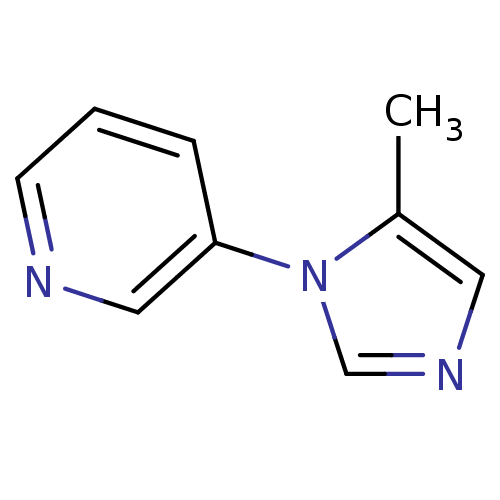
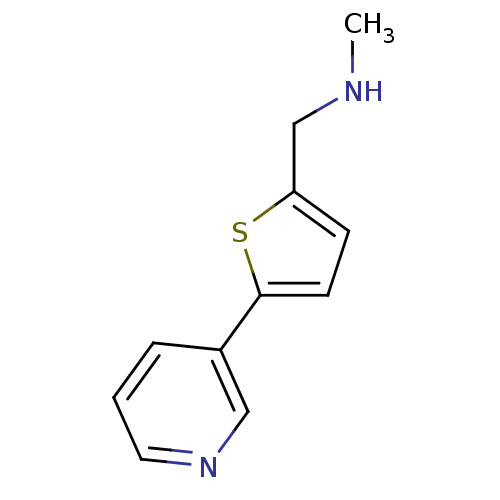
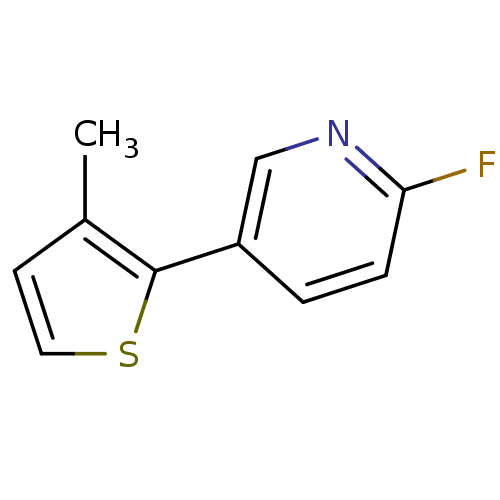
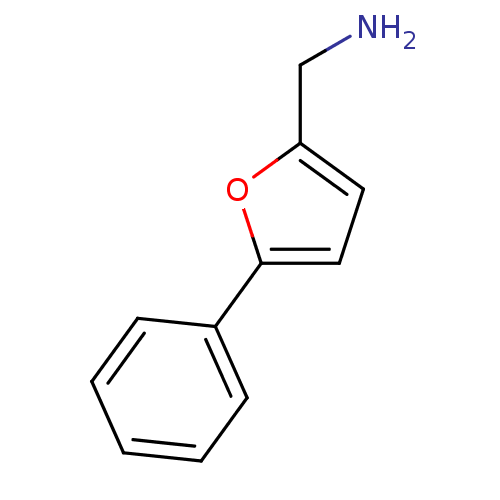
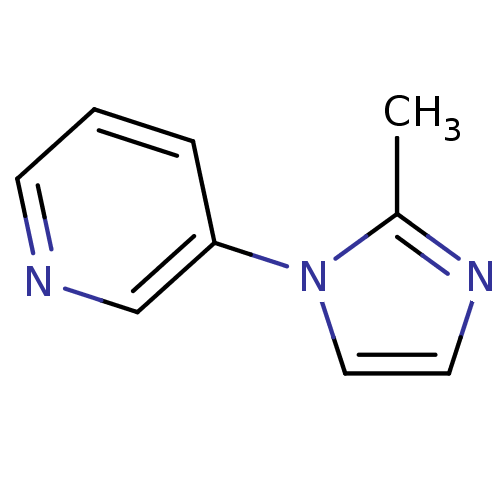
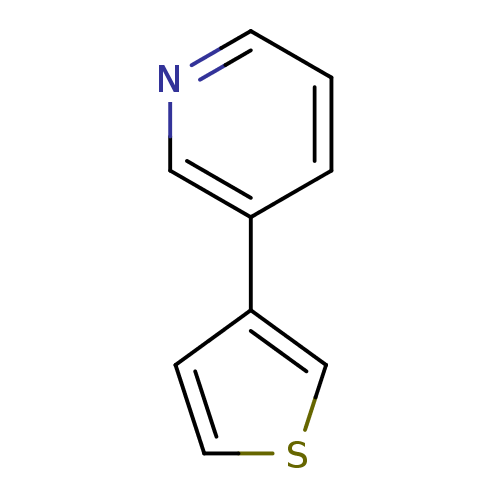
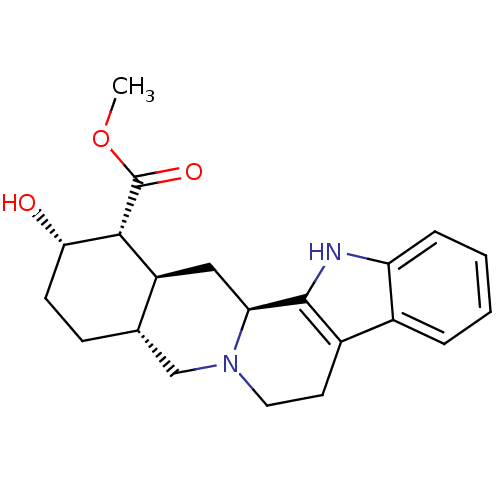
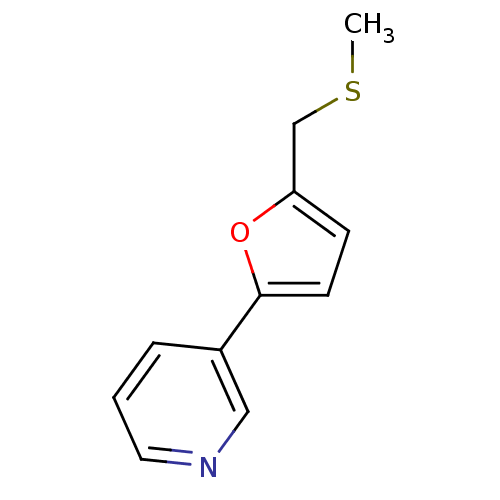
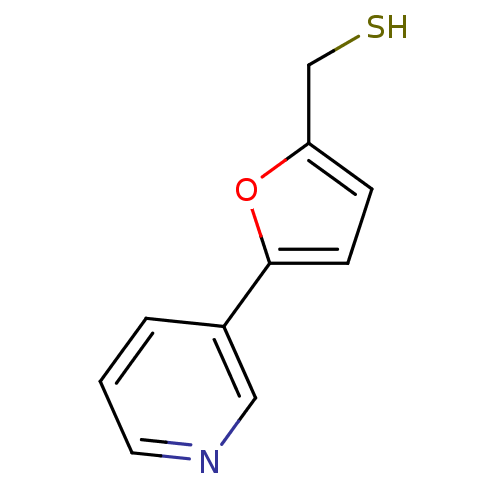
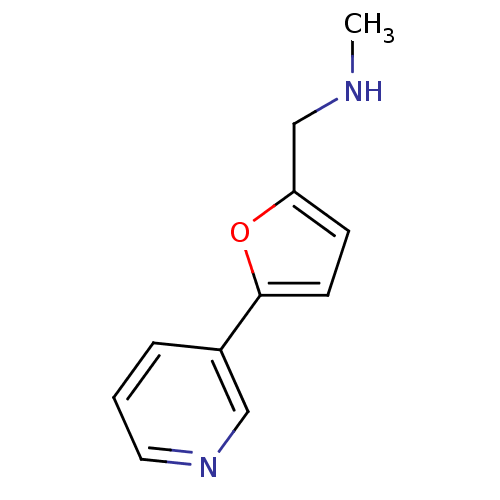
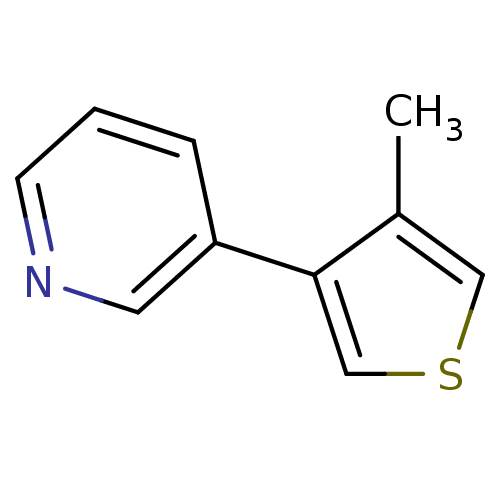
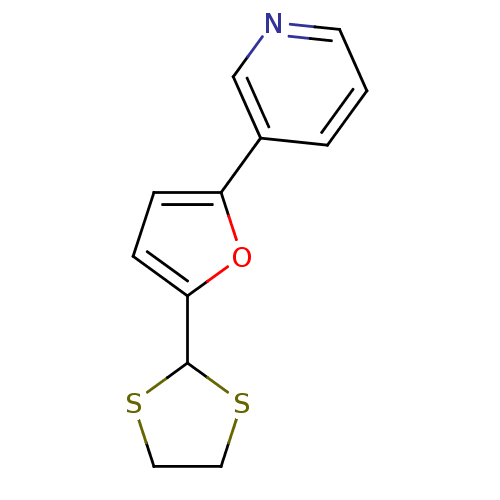
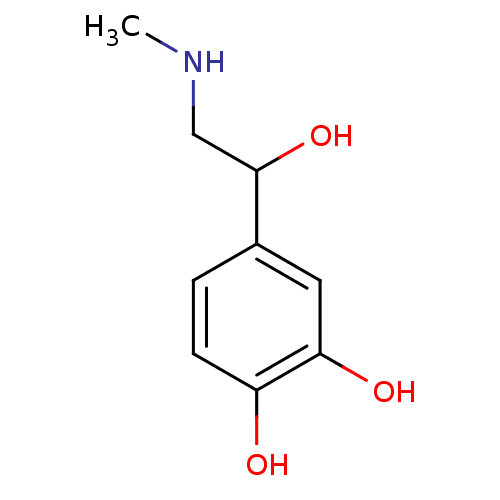
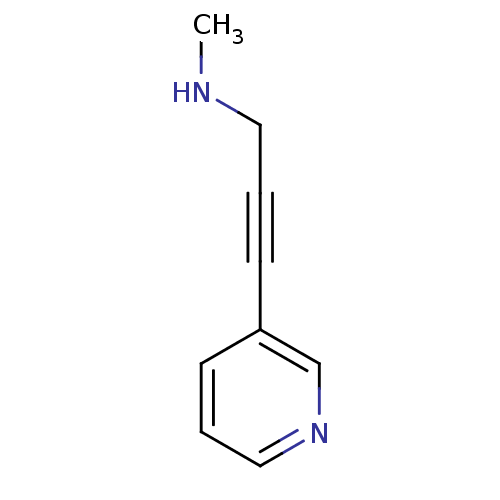
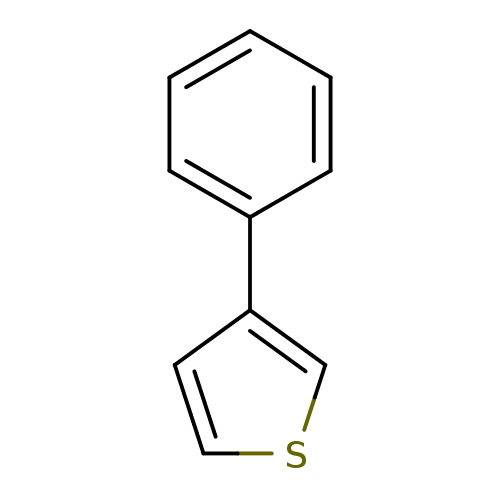
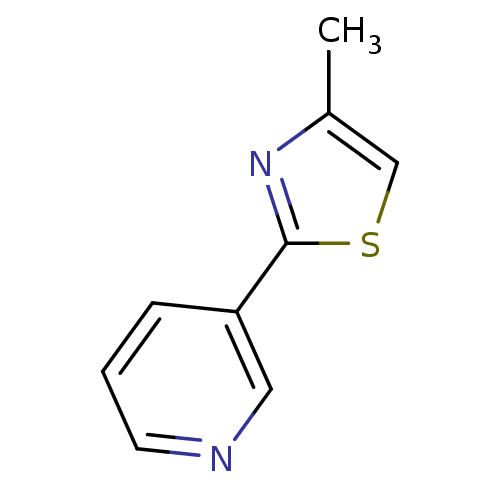
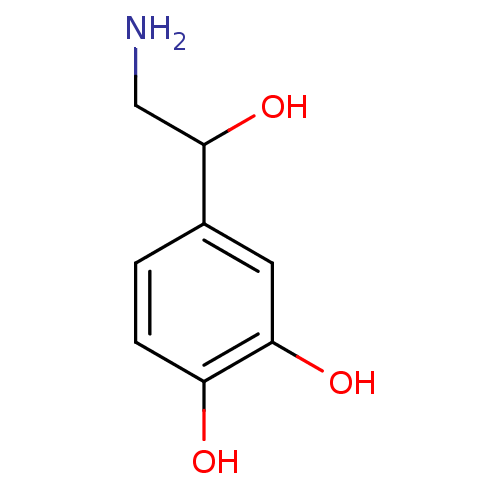
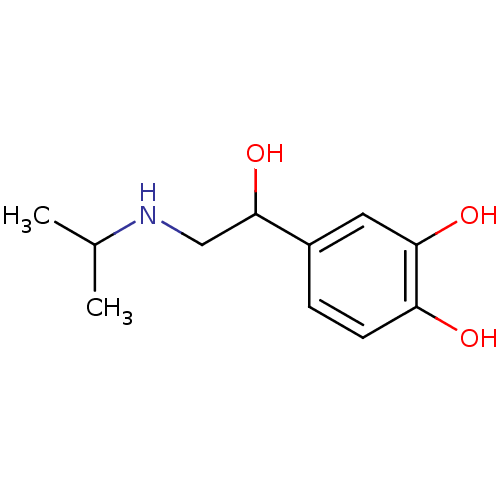
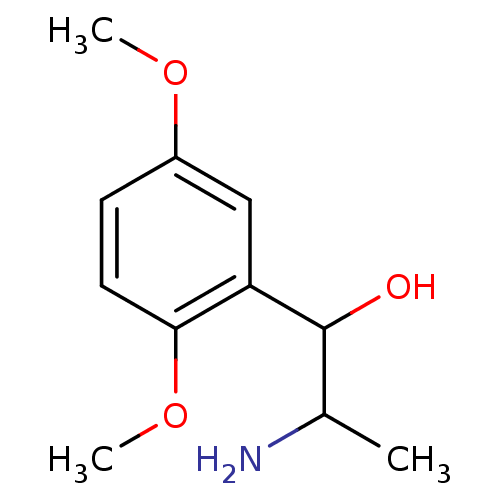
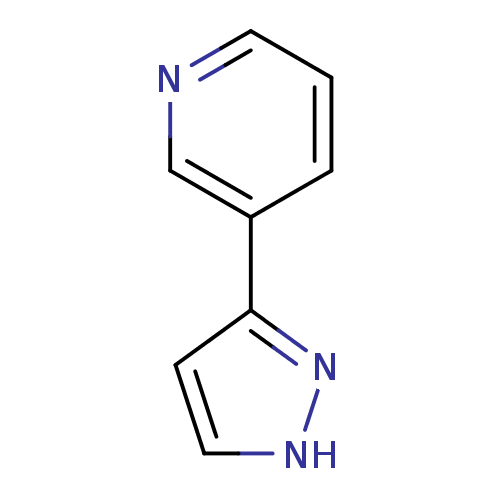
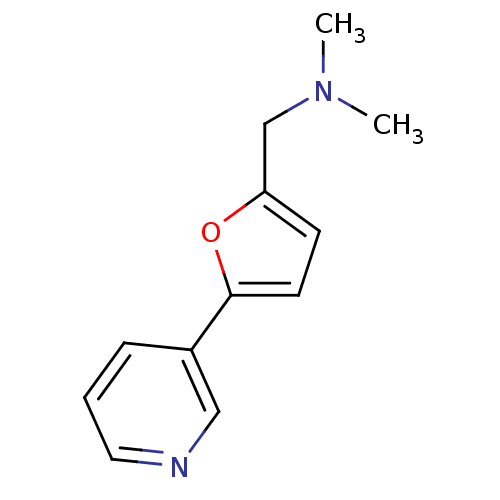
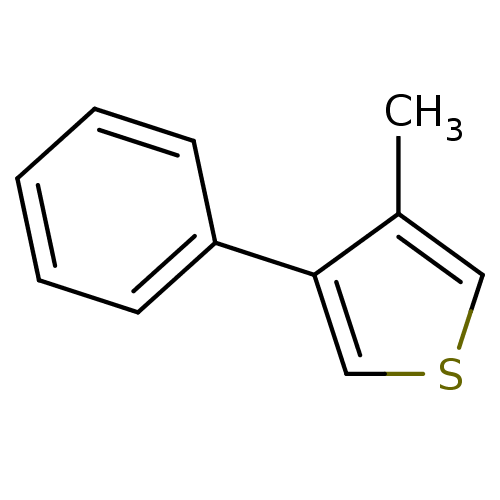TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
Affinity DataKi: 80nM ΔG°: -42.1kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 100nM ΔG°: -41.6kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -38.7kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -38.7kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 400nM ΔG°: -38.0kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 500nM ΔG°: -37.4kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 600nM ΔG°: -36.9kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 600nM ΔG°: -36.9kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 700nM ΔG°: -36.5kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 700nM ΔG°: -36.5kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
Affinity DataKi: 800nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: <800nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 800nM ΔG°: -36.2kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
Affinity DataKi: 2.70E+3nM ΔG°: -33.1kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 3.30E+3nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 4.10E+3nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
Affinity DataKi: 6.20E+3nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 7.40E+3nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
National Children'S Medical Research Center
Curated by PDSP Ki Database
National Children'S Medical Research Center
Curated by PDSP Ki Database
Affinity DataKi: 1.28E+4nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 1.39E+4nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 1.42E+4nM ΔG°: -28.8kJ/molepH: 7.5 T: 2°CAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataKi: 1.52E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 1.96E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 2.29E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 2.36E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: <2.50E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:To measure CYP3A4 activity, testosterone 6-hydroxylation was determined. After reactions were terminated, the organic phase was collected and removed...More data for this Ligand-Target Pair

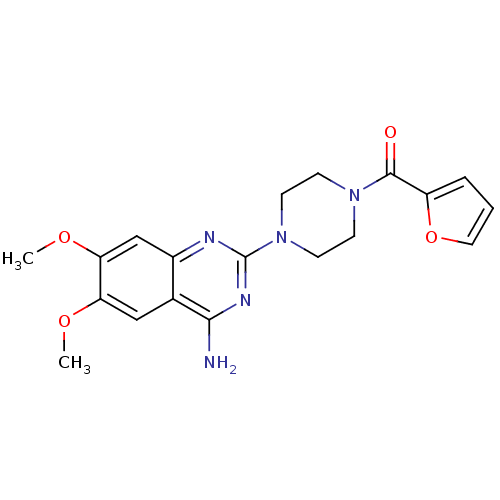



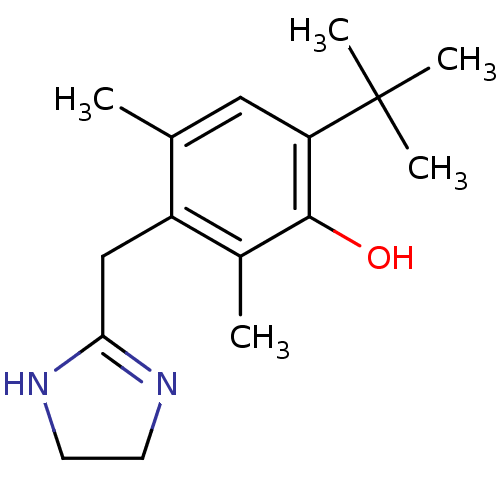
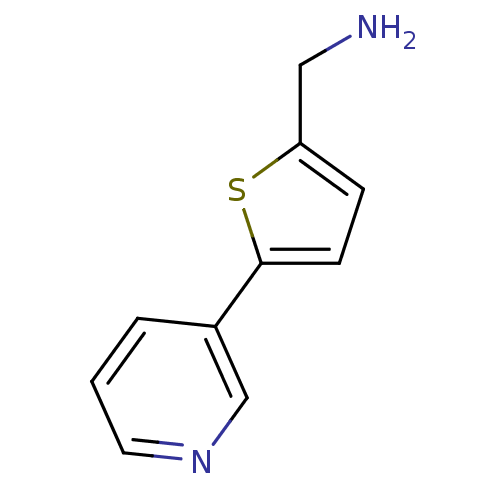
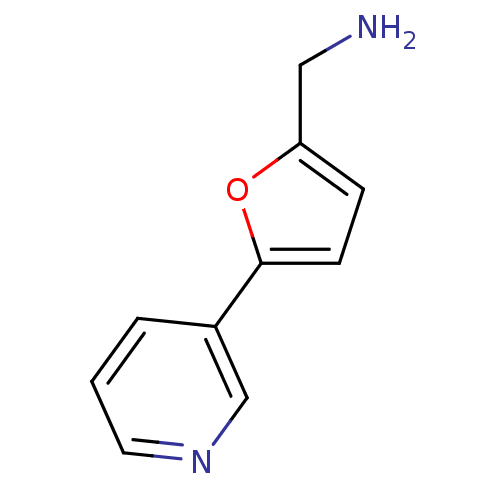
 3D Structure (crystal)
3D Structure (crystal)